| Extracellular heme proteins influence
bovine myosatellite cell |
Primary bovine satellite cells (BSCs) from
semitendinosus of Charolaise × Simmental beef cow |
The proliferation and metabolic activity
of BSCs was significantly increased when myoglobin (Mb) was added.
Mb application to bioartificial muscles led to a the development of
a color similar to that of the cooked beef. |

|
Simsa et
al. (2019) with CC-BY |
| Serum-free media for the growth of bovine
myoblasts |
Skeletal muscle cell of cow biceps
femoris
|
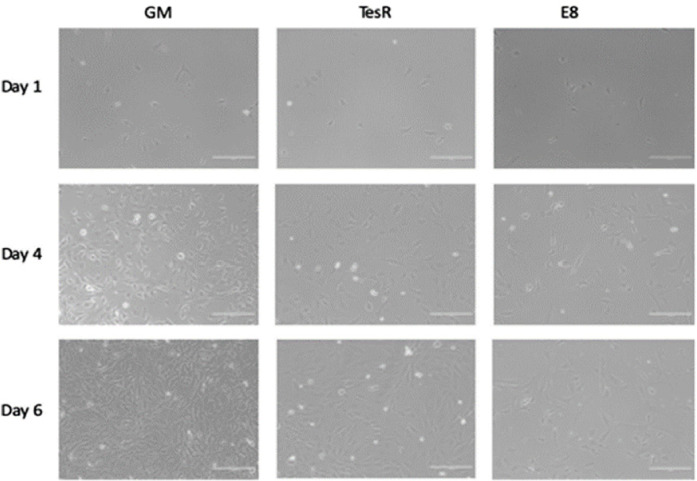
Serum-free media stimulate exponential
cell expansion, albeit not to the extent of the current growth
medium containing up to 30% serum. Further research is needed
to investigate whether prolonged cell culture or an adaptation
period could further increase cell proliferation. |
|
Kolkmann
et al. (2020) with CC-BY |
| Simple and effective serum-free medium for
sustained expansion of bovine satellite cells for cultured meat
production |
Primary bovine satellite cells |
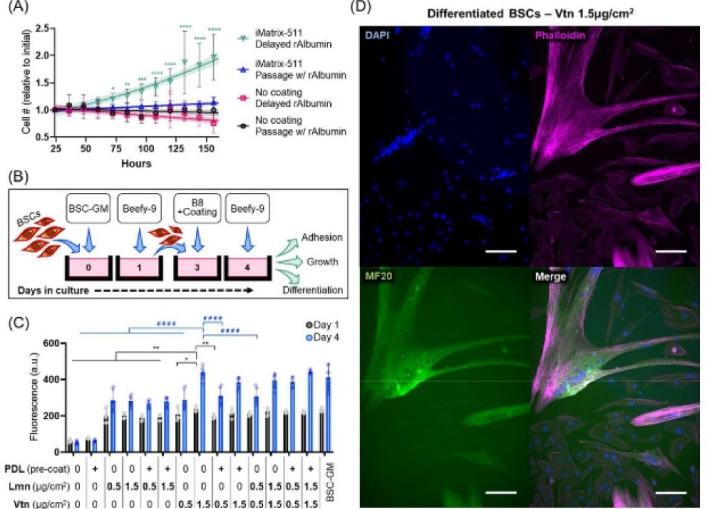
This new media (Beefy-9) maintained robust
cell growth over the entire culture period tested (seven passages)
with an average growth rate of 39 hours per population
doubling. |
|
Stout et
al. (2022) with CC-BY |
| Effect of smooth muscle cells on the
quality of cultured meat |
Smooth muscle cells of piglet |
The addition of basic fibroblast growth
factor to the medium significantly increased the growth rate of
smooth muscle cells and the expression of extracellular
matrix-related genes, especially collagen and elastin. |
|
Zheng et
al. (2021)
|
| Taste characteristics of satellite cell
cultured meat |
Chicken skeletal muscle cell |
The content of all amino acids except
valine and tyrosine was significantly different between cultured
meat and traditional meat. |
|
Joo et al.
(2022) with CC-BY-NC |
| Proliferation and differentiation of
bovine myoblasts using Chlorella vulgaris for
cultured meat |
Primary bovine myoblasts (PBM) |
The addition of Chlorella
vulgaris extract (CVE) significantly improved PBM
viability compared to that in conventional culture medium.
Furthermore, by adding horse serum to induce differentiation, the
formation of myotubes was confirmed when CVE was used. |
|
Okamoto et
al. (2022)
|
| Bovine satellite cell maintains the
proliferative myogenic capacity for cultured meat |
Satellite cell of Holstein M.
semimembranosus
|
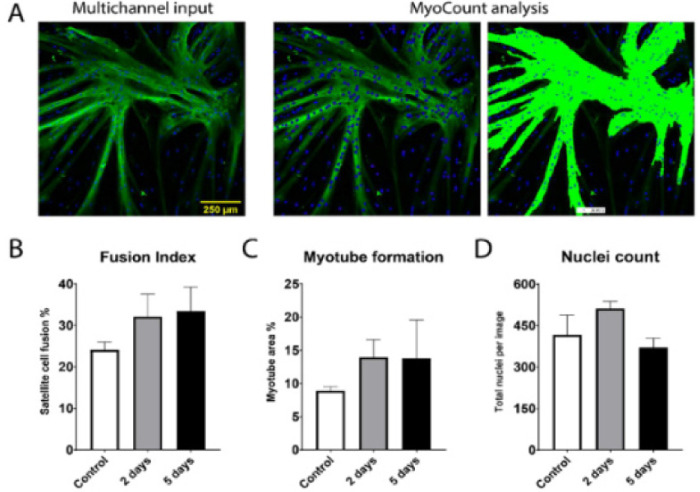
The data indicated a positive trend in
terms of myogenic potential after tissue storage. The timeframe in
which viable myogenic satellite cells can be isolated and used for
cultured meat production can be greatly extended by proper tissue
storage. |
|
Skrivergaard et al. (2021) with CC-BY |
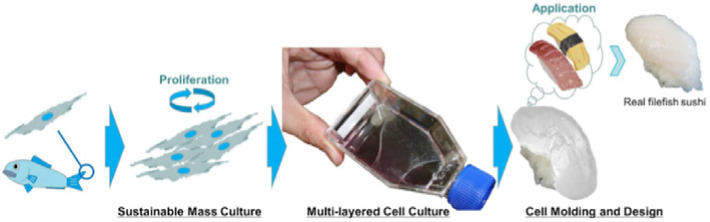
| Develop aquatic clean meat from fish
cells |
Fibroblast-like cell of the fin of
thread-sail filefish (Stephanolepis cirrhifer) |
Cell differentiation was regulated by a
“simple stimulus” such as medium, serum and
extracellular matrix without using a specialized technique. |
|
Tsuruwaka
and Shimada (2022) with CC-BY |
| Multi-layered skeletal muscle tissue by
using 3D collagen scaffolds |
Rat L6 skeletal muscle myoblasts |
3D micropatterned scaffolds can promote
cell alignment and muscle tissue formation. The micro-grooved
collagen scaffolds could be used to engineer organized multi-layered
muscle tissue. |
|
Chen et
al. (2015)
|
| Developing cultured meat scaffolds of
vegetable-based proteins |
C2C12 skeletal muscle cells |
Fibrous growth substrates from extruded
plant-based proteins that the cells are able to attach to and grow
on. |

|
Krona et
al. (2017)
|
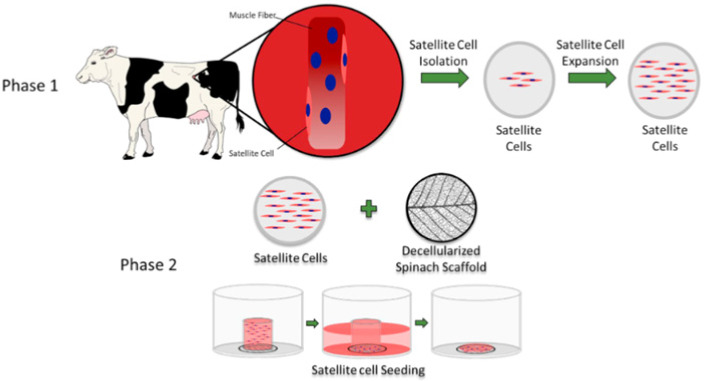
| Edible scaffold (decellularized spinach)
for cultured meat |
Bovine satellite cell |
After 14 d, primary bovine satellite cells
seeded on the decellularized leaf scaffold maintained approximately
99% viability, and approximately 25% of the cells
expressed the myosin heavy-chain. |
|
Jones et
al. (2021) with CC-BY-NC-ND |
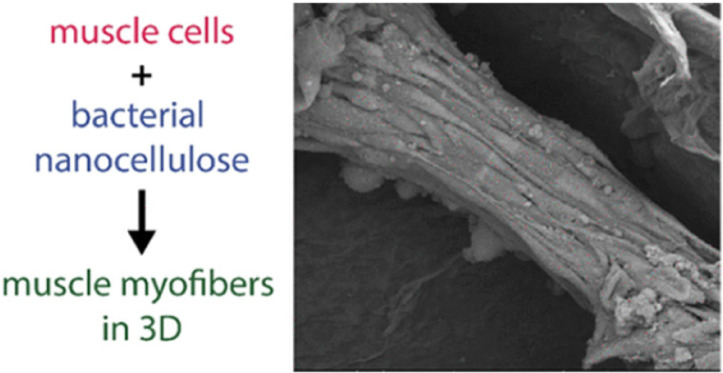
| Nanocellulose from Nata de
Coco as a bioscaffold for cell-based meat |
Mouse C2C12 myoblast |
Nanocellulose bioscaffolds show limited
potential as a biocompatible matrix for cell-based meat. |
|
Rybchyn et
al. (2021) with CC-BY-NC-ND |
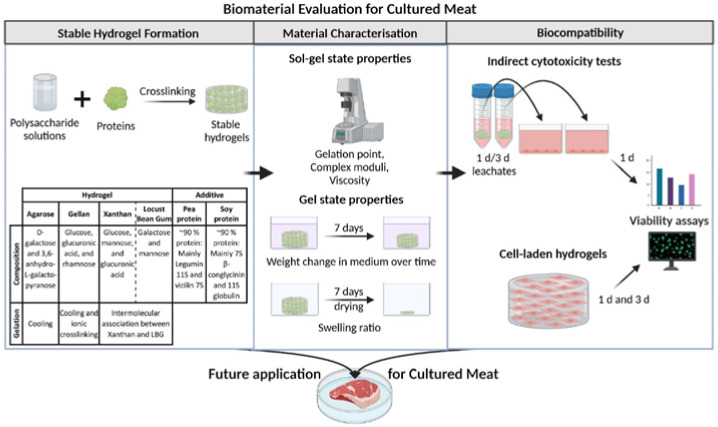
| Scaffolds for cultured meat on
the basis of polysaccharide hydrogels with plant-based protein |
Murine myoblast C2C12
cell |
All evaluated polysaccharide-protein
blends turned out as potential candidates for cultured meat. |
|
Wollschlaeger et al. (2022) with CC-BY |
| It is possible to make protein blends
(containing up to 1% of pea and soy protein) with all
polysaccharides to increase the nutritional value. |
| Chitosan-collagen hydrogel microparticles
for cultured meat |
Mouse C2C12 skeletal myoblasts |
Cell microcarriers support the attachment
and rapid proliferation of mouse skeletal C2C12 myoblasts, rabbit
smooth muscle cells, sheep fibroblasts, and bovine umbilical cord
mesenchymal stem cells. |
|
Zernov et
al. (2022)
|
| Modified cell-electrospinning for 3D
myogenesis of C2C12 |
C2C12 myoblasts |
Loading C2C12s as cellular aggregates and
modifying several other electrospinning parameters drastically
increased cell viability. C2C12-seeded fibrin/polyethylene oxide
microfiber bundles were cultured for up to 7 d. |
|
Guo et al.
(2019)
|
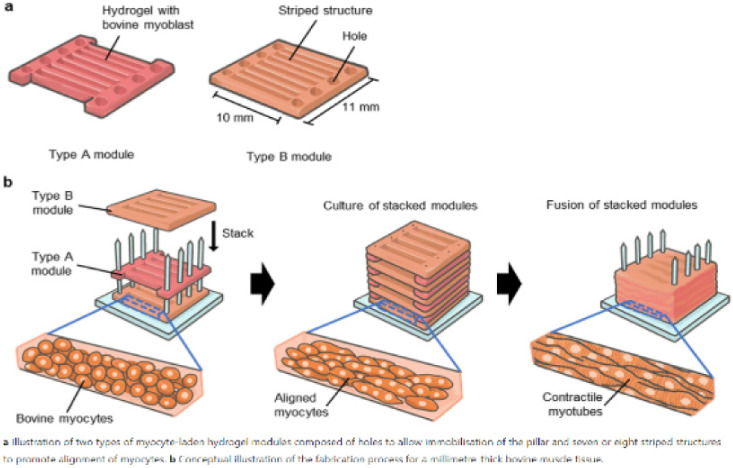
| Formation of contractile 3D bovine muscle
tissue for construction of millimeter-thick cultured steak |
Bovine myocytes of beef cattle |
When the myocytes were cultured in the
hydrogel for 14 d, fiber-shaped bovine muscle tissue of diameter
295±105 μm was generated, the ends of which were
immobilized with pillars, showing that the length of the muscle
tissue was equal to the gap between the anchors (7 mm). |
|
Furuhashi
et al. (2021) with CC-BY |
| Cultured meat production using 3D printing
technology |
Newborn pig satellite cell |
The 4% sodium alginate-gelatin and
gelatin-methacrylate 20% silk fibroin hydrogel demonstrated
good performance and was hybridized with porcine skeletal muscle
satellite cells for 3D printing. |

|
Li et al.
(2021) with CC-BY-NC-ND |
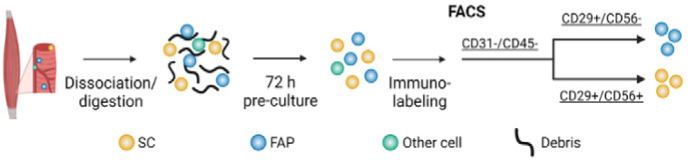
| Muscle-derived fibroadipogenic progenitor
(FAP) cell for production of cultured bovine adipose tissue |
FAP cells |
FAP cells reached a mature level of
adipogenic differentiation in three-dimensional, edible hydrogels.
The resultant tissue accurately mimics traditional beef fat, and FAP
cells thus represent a promising candidate cell type for the
production of cultured fat. |
|
Dohmen et
al. (2022) with CC-BY |













