Abstract
Objective:
Disruptions in cognition are a clinically significant feature of bipolar disorder (BD). The effects of different treatments on these deficits, and the brain systems that support them, remain to be established.
Method:
Participants, including 55 healthy individuals and 71 acutely ill youth with mixed/manic BD, performed a continuous performance test to assess vigilance and working memory during task-based fMRI studies. Patients, who were untreated for at least seven days at baseline, and healthy individuals were scanned at pre-treatment baseline and at week one and six. After baseline testing, patients (n = 71) were randomized to six-week double-blind treatment with lithium (n = 26, 1.0–1.2 mEq/L) or quetiapine (n = 45, 400–600 mg). Weighted seed-based connectivity (wSBC) was used to assess regional brain interactions during the attention task compared to the control condition.
Results:
At baseline, youth with BD showed reduced connectivity between bilateral anterior cingulate cortex (ACC) and both left VLPFC and left insula, and increased connectivity between left VLPFC-left temporal pole, left OFC-right postcentral gyrus and right amygdala-right occipital pole compared to healthy individuals. At week one follow-up, quetiapine but not lithium treatment led to a significant shift of connectivity patterns toward those of the healthy participants. At week six, compared to baseline, there was no difference between treatment conditions, at which time both patient groups showed significant normalization of brain connectivity toward that of healthy individuals.
Conclusion:
Functional alterations in several brain regions associated with cognitive processing and the integration of cognitive and affective processing were demonstrated in untreated youth with BD prior to treatment. Treatment reduced several of these alterations, with significant effects at week one only in the quetiapine treatment group. We conclude that normalization of functional connectivity might represent a promising biomarker for early target engagement in youth with BD.
Keywords: bipolar disorder, treatment, lithium, quetiapine, fMRI
Introduction
Bipolar disorder (BD) is a complex, common, and disabling psychiatric disorder affecting 1.5–3% of the population worldwide. Many BD patients experience their first episode of illness during adolescence.1,2 While neuropsychological impairments are well established to be associated with BD, little is known about the neural mechanisms of these deficits in young patients with BD and how they are affected by treatment.
Multiple cognitive deficits have been established in both adults and youth with bipolar disorder including impairments of attention and working memory.3,4 Impairments in functional brain circuitry supporting attention5 and working memory6,7 have also been reported in BD functional magnetic resonance imaging (fMRI) studies8–10. Findings in studies assessing these features include reduced activity of the dorsal attention system, and while other studies, especially using affective tasks, have also demonstrated hyperactivity of the limbic system suggesting hyperarousal of affective and interoceptive brain systems.11 Other studies have shown that neural systems supporting attention and working memory performance may be more disrupted by stimuli eliciting emotional responses than in healthy individuals12,13, and that youth with BD have high rates of comorbid attention problems including ADHD.14 While these studies have contributed to the understanding of regional functional brain deficits in pediatric BD, almost all fMRI studies of youth with BD focused on local brain activation, and the nature of connectivity alterations between affective and attention hubs and other brain regions remains relatively unexplored,15,16 especially in children and with regard to the effect of treatment on the functional brain connectome.
Lithium, one of the oldest treatments for mania, and quetiapine, a second-generation antipsychotic (SGA), are two widely used and effective interventions for mania in youth with BD.17,18 Previous study examined the risks and benefits of pharmacotherapy of patients with BD presenting with an acute manic or mixed episode and revealed greater and earlier efficacy of SGAs when compared to lithium in controlling manic symptoms.19 Though quetiapine and lithium exert effects via different mechanisms, there are few neuroimaging studies to understand their systems level impact on the brain connectome, their differences in location and timing of such effects, and their relation to treatment outcomes in vivo.20,21 In adult patients with BD presenting with an acute manic episode, a resting-state fMRI study found that lithium treatment showed a more rapid normalization with respect to abnormal functional connectivity observed at baseline.22 While pediatric connectome studies of drug effects in BD are limited, difference in regional effect of the antipsychotic risperidone and divalproex have been reported in children with BD presenting with an acute manic or mixed episode.23
Because BD not uncommonly has an onset before adult years, developmental considerations in both behavior and brain anatomy and function through adolescence require studies of children and adolescents that investigate treatment related changes in brain function in this population.24,25 To our knowledge, there has been no randomized, double-blind, controlled study to compare treatment effects of lithium and quetiapine on the functional systems related to cognitive processing using fMRI in manic youth with BD.
In light of our current understanding of the abnormalities of brain function in BD,8,26 we hypothesized youth with BD before treatment would exhibit abnormal connectivity in brain regions implicated in attentional processing, that hyper-reactivity of brain affective systems would be seen during cognitive task performance. Second, we hypothesized that pharmacologic treatments would alleviate the abnormal functional connectivity observed found at baseline, and that these changes would parallel clinical improvement during the trial. We also tested for differential effects of lithium and quetiapine in the speed and extent of normalization of functional connectivity alterations, and whether pretreatment fMRI measures could predict clinical outcomes for either treatment.
Method
Participants
This study (clinicaltrials.gov identifier: NCT00893581) enrolled manic or mixed youths with bipolar disorder type-I (BD I). This study, (NCT00893581), Multimodal Neuroimaging of Treatment Effects in Adolescent Mania,19 included two aims reported which are the focus of this paper: (1) To determine the differential effects of treatment with quetiapine or lithium on brain activation in children and adolescents with mixed/manic BD using fMRI, and (2) To determine the relationships between the changes in brain activation and symptom improvement resulting from the two different therapies. Diagnoses of bipolar I disorder, mixed/manic phase, were confirmed by trained raters with established diagnostic inter-rater reliability (kappa > 0.9) via administration of the Washington University in St. Louis Kiddie Schedule of Affective Disorders and Schizophrenia (WASH-U-KSADS).27 Mood symptoms were rated using the Young Mania Rating Scale (YMRS),28 Children’s Depression Rating Scale-Revised (CDRS-R),29 and Clinical Global Impressions-Severity (CGI-S).30 Patients included met the following criteria: (1) experiencing a manic or mixed episode; (2) had a baseline YMRS score ≥ 20; (3) were less than two years from onset of BD as defined by their first mood episode; (4) had no prior psychiatric hospitalizations for mania; (5) had no history of treatment with therapeutic doses of antipsychotic drugs, mood stabilizers or antidepressants for > three months, and no psychotropic medication during the week (72 hours for psychostimulants) prior to baseline MRI and clinical assessment.
Demographically matched healthy youths were recruited from outreach programs in communities where the bipolar participants resided. All bipolar and healthy subjects met the following inclusion criteria: (1) age 10–17 years 11 months and at Tanner stage II-V;31 (2) no history of substance dependence; (3) no alcohol or drug abuse for at least three months prior to the scan; (4) no history of mental retardation or documented IQ < 70 as measured by the Wechsler Abbreviated Scale of Intelligence (WASI);32 (5) right-handed; (6) no history of major systemic or neurological disorders likely to influence fMRI results; (7) no contraindication for an MRI study; (8) ability to communicate in English; and (9) if female, a negative pregnancy test. All participants provided written assent and their legal guardians provided written informed consent for this study after the procedures and risks were explained in full. Both the University of Cincinnati and Children’s Hospital Medical Center Institutional Review Boards approved this study. Participants were enrolled in the study by a research coordinator (CCK) if they passed screening of these inclusion/exclusion criteria following the Consolidated Standards of Reporting Trials (CONSORT) guidelines for the registered randomized clinical trials.
Treatment Procedures
Following baseline clinical evaluation and scanning, patients were randomized, by an investigational pharmacist, to double-blind treatment with quetiapine or lithium and evaluated weekly for six weeks. The randomization schedule was stratified by presence vs. absence of ADHD, presence vs. absence of psychosis, and mood state (mixed vs. manic episode). Treatment response was defined as a ≥ 50% reduction in YMRS scores from baseline.33
Quetiapine was initiated at 100 mg/day and lithium carbonate was initiated at 30 mg/kg (maximum starting dose of 600 mg twice daily). Patients were also given placebo capsules for the medication to which they were not assigned. Quetiapine/placebo and lithium/placebo capsules were identical. Quetiapine was titrated to a target dose of 400–600 mg daily based on tolerability and response. Lithium was titrated to a serum level of 1.0–1.2 mEq/L. Treatment was administered in a double-dummy double-blind manner, with an unblinded study physician monitoring trough lithium levels and making dose adjustments independent from treating physicians and clinical raters. However, blinded clinical rater dose tolerability adjustment recommendations took precedence over unblinded physician double dummy dose adjustment recommendations. The YMRS was used to assess symptom severity before and throughout the trial.
Behavioral Paradigm
Participants were presented with a series of single-digit numbers (one per 700 ms with a 50 ms gap between them) and were asked to respond with a button press when the same number occurred twice sequentially (Figure S1, available online). Responses were recorded to permit calculation of response parameters (i.e., discriminability, percent correct and reaction time). This is essentially a 1-back working memory task. The control module consisted of the number ‘1’ presented at the same rate as the active task, during which subjects were asked to press the response button for the first five stimuli and then watch the remainder of the control task presentation without responding (Figure S1, available online). This task was designed to control for effects of watching visual stimuli when monitoring them for repetition was not required. The steady task demands through blocks permitted functional connectivity throughout the block to be evaluated.
Subjects were presented stimuli using nonferromagnetic goggles (Resonance Technologies Inc.). Experimental and control tasks were administered in alternating blocks of 30 s each with numbers being presented for 700 ms every at 750 ms, providing a 50 ms gap between presentations, for a total of 40 numbers/block. Five blocks of the active task and six control blocks were presented during each scan session.
Image Acquisition
All subjects were scanned using a 4.0 Tesla Varian Unity INOVA MRI scanner. Prior to beginning the study, subjects could familiarize themselves with the scanner to decrease any anxiety. Subjects completed anatomical T1-weighted, 3-D brain scans first and then completed an fMRI session in which scans were acquired while performing the CPT-IP task using a T2*-weighted gradient-echo EPI pulse sequence. The image acquisition details were provided in the Supplement 1, available online.
All participants were scanned three times: at baseline, week one and week six. After excluding participants who did not complete all three scans and clinical evaluations, we excluded additional participants that had excessive head motion during MRI scans (two patients with BD receiving lithium treatment, two patients with BD receiving quetiapine treatment and one healthy individual), a total of 71 patients with BD (45 patients receiving quetiapine monotherapy and 26 patients receiving lithium carbonate monotherapy) and 55 healthy individuals were included in statistical analyses.
fMRI Data Analysis
Details of preprocessing of fMRI data were provided in the Supplement 1. In the first-level analysis, weighted seed-based connectivity (wSBC) analysis was used to characterize the condition-specific functional connectivity strength before and after treatment. Compared to the traditional seed-based functional connectivity analysis usually used in resting-state fMRI studies, wSBC uses condition-specific weights defined from the hemodynamic response function. The wSBC was analyzed using atlas-based seed regions believed to be involved in BD pathology, including bilateral amygdala, ventral and dorsal lateral prefrontal cortex (VLPFC and DLPFC), orbital frontal cortex (OFC) and insula34 using the WFU_PickAtlas MATLAB toolbox (https://www.nitrc.org/projects/wfu_pickatlas), leading to 10 separate seeds for connectivity analysis. The wSBC map was generated by computing condition-specific Pearson’s correlation coefficients between the BOLD time series of the seed and the BOLD time series of voxels outside the seed using a general linear model (GLM). Weighted SBC maps can be interpreted exactly in the same way as standard SBC maps, only restricted to the duration of one specific task or condition (wSBC are defined so that they are exactly equal to SBC maps when using constant weights encompassing the entire time series). The connectivity difference between the active task and the control condition was obtained by subtracting the wSBC during the two conditions. See Supplement 1 for more details of this method.
Then, connectivity maps of all each seed were analyzed. Baseline wSBC maps of connectivity of each seed region with other gray matter voxels were compared between patients and healthy individuals, with age and sex considered as covariates. Regions exhibiting group differences were reported if they survived a voxel-height threshold at uncorrected p < 0.001 and a cluster size FDR-corrected p < 0.05. Significant clusters identified at baseline were then used as masks for the longitudinal analysis to characterize the drug effect on identified pretreatment connectivity abnormalities.
Statistical Analysis
To study longitudinal changes of functional connectivity after over the course of medication treatment, we extracted connectivity values from the wSBC maps at baseline, week one and week six. Group-by-time interaction effects were tested using a random-effect analysis of variance (ANOVA) model. Normalization of connectivity in youth with BD was defined using the connectivity of typically developing youth as the reference. The longitudinal within-group connectivity changes were tested using a general linear model (GLM). To identify different treatment outcomes from lithium and quetiapine, we first added group (lithium, quetiapine and healthy groups) as a factor in the GLM. Because treatment effects may be shared or distinct across the two treatments, if our model failed to identify significant differences in connectivity changes between the treatment groups, we combined the two treatment groups (lithium and quetiapine groups) to examine case control differences over time. We used multivariable linear regression analysis to characterize the relationship between normalization in functional brain connectivity and rate of improvement in mania symptoms, and to determine whether baseline abnormal connectivity predicted mania symptom change over the course of treatment. Age and sex were considered as covariates in all correlation analyses. Statistical analyses were performed using R software (Version 3.6.0).
Results
Demographic and Clinical Data Results
Demographic and clinical characteristics for both groups are listed in Table 1. The patients and healthy individuals were comparable at baseline in age and sex. However, the patient group had a lower IQ compared with healthy individuals (t = 2.97, p = 0.004). In the BD group, patients in the lithium and quetiapine treatment groups were comparable in all demographic metrics. From our earlier clinical research, we found that quetiapine was associated with a statistically significant greater rate of response and overall symptom reduction compared to lithium in youth with BD in this sample.19 When comparing treatment effects of lithium and quetiapine, we found significant treatment-by-time effects for baseline to week 1 YMRS changes (F = 4.73, p = 0.032, Figure S2, available online). Post hoc analyses showed that compared with the lithium group, the patients in the quetiapine group showed a greater YMRS decrease when controlling for baseline YMRS scores (F = 5.18, p = 0.025). This kind of interaction effect was not significant for baseline to week 6 changes for the two drugs, or when the model included data from all three points (p > 0.05).
Table 1.
Demographic and Clinical Information for Study Participants
| BD (n=71), Mean ± SD | p-value (Li vs. Que) | HC (n=55), Mean ± SD | p-value (BD vs. HC) | ||
|---|---|---|---|---|---|
|
|
|||||
| Lithium group (n=26) | Quetiapine group (n=45) | ||||
| Age, years | 14.9 ± 1.9 | 14.0 ± 1.9 | 0.058 | 14.8 ± 1.9 | 0.203 |
| Gender, female/male | 17/9 | 29/16 | 1.000 | 30/25 | 0.326 |
| IQ | 104.5 ± 11.8 | 103.42 ± 12.2 | 0.721 | 110.5 ± 12.9 | 0.004 |
| YMRS | 27.2 ± 5.3 | 27.3 ± 5.0 | 0.978 | 0.5 ± 1.1 | <0.001 |
| CDRS | 38.1 ± 7.6 | 37.2 ± 9.6 | 0.644 | 17.5 ± 0.9 | <0.001 |
| CGI severity overall | 5.0 ± 0.5 | 4.9 ± 0.6 | 0.405 | 0 ± 0 | <0.001 |
| CGI severity depression | 4.4 ± 1.0 | 4.3 ± 1.1 | 0.842 | 0 ± 0 | <0.001 |
| CGI severity mania | 4.8 ± 0.5 | 4.7 ± 0.7 | 0.225 | 0 ± 0 | <0.001 |
Note: BD = bipolar disorder; CDRS = Children’s Depression Rating Scale; CGI = Clinical Global Impression; HC = healthy controls; YMRS = Young Mania Rating Scale.
Task Performance Results
Task performance for all participants was recorded (see Table S1, available online). At all three time points, patients showed poorer CPT-IP task performance (i.e., lower percent correct and discriminability) compared with healthy individuals (p < 0.05). Longitudinal analyses showed no significant improvement over time (reflected in group-by-time interactions) in these cognitive measures in either treatment group relative to healthy individuals (p > 0.05).
wSBC Alterations Before Treatment
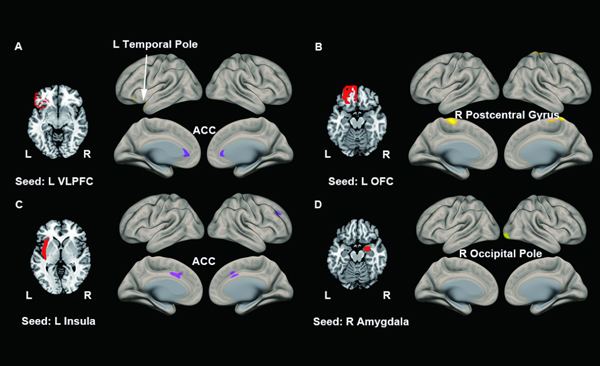
Figure 1 illustrates areas of significant regional connectivity differences in healthy vs. bipolar study participants while performing the CPT-IP task before treatment. We found significantly increased connectivity between left VLPFC and left temporal pole (p = 0.037), left OFC and right postcentral gyrus (p = 0.010) and right amygdala and right occipital pole (p = 0.005), and decreased connectivity between left VLPFC (p = 0.024) and left insula (p = 0.007) with bilateral anterior cingulate cortex (ACC) in patients compared to healthy individuals (Table 2). After using the conservative Bonferroni correction for multiple comparisons, only the connectivity between right amygdala and right occipital pole (p = 0.005) survived the correction.
Figure 1. Abnormal Connectivity in Patients With Bipolar Disorder (BD) Compared to Healthy Individuals.
(A) using the L VLPFC as the seed, we found increased L VLPFC-L temporal pole connectivity and decreased L VLPFC-ACC connectivity. (B) using the L OFC, we found increased L OFC-R postcentral gyrus connectivity. (C) using the L insula as the seed, we found decreased L insula-ACC connectivity. (D) using the R amygdala as the seed, we found increased R amygdala-R occipital pole connectivity. ACC = anterior cingulate cortex; L = left; OFC = orbital frontal cortex; R = right; VLPFC = ventrolateral prefrontal cortex.
Table 2.
Baseline Frontal Cortex (FC) Differences Between Bipolar Disorder (BD) and Healthy Controls
| Seeds | Clusters | Size | MNI Coordinate for the peak | Cluster-size FDR corrected p-value |
|---|---|---|---|---|
| LVLPFC | L Temporal Pole | 311 | [−48, 20, −20] | 0.037 |
| ACC | 434 | [0, 34, 4] | 0.024 | |
| L OFC | R Postcentral Gyrus | 532 | [12, −40, 74] | 0.010 |
| L Insula | ACC | 586 | [0, 16, 38] | 0.007 |
| R AMY | R Occipital Pole | 583 | [26, −92, −12] | 0.005 |
Note: ACC = anterior cingulate cortex; AMY = amygdala; L = left; R = right; OFC = orbital frontal cortex; VLPFC = ventrolateral prefrontal cortex.
wSBC Changes After Medication Treatment
To study changes after one and six weeks of treatment, we used three models: one with only the first two points in time (baseline and week one) to identify early changes in regions with pre-treatment connectivity alterations, and one with baseline and week six data to study net change over the trial). We also report findings from the model including all three points in time (baseline, week one and six). For the model including baseline and week one data, we found significant differences among the lithium, quetiapine, and healthy groups in change of connectivity from baseline between left VLPFC and left temporal pole (F = 3.26, p = 0.042) and between left OFC and right postcentral gyrus (F = 3.45, p = 0.035). Post-hoc Tukey tests showed that the quetiapine group showed decreased connectivity between left VLPFC and left temporal pole (p = 0.032) and decreased connectivity between left OFC and right postcentral gyrus (p = 0.030) relative to controls. These changes represented a normalizing effect towards FC of healthy individuals after only one week of drug treatment. There were no significant differences of one-week connectivity changes between the lithium and healthy group or between the quetiapine and lithium group (p > 0.05) (Figure 2A).
Figure 2. Connectivity Changes From Baseline to Week 1 and Week 6 During Drug Treatment.
(A) one-week changes of L VLPFC-L temporal pole connectivity and L OFC-R postcentral gyrus connectivity in the quetiapine, lithium, and healthy group. The quetiapine group showed significant normalization relative to the control group while the lithium group did not show this effect. (B) Six-week changes from baseline of L insula-ACC and right amygdala and right occipital pole connectivity in patients and healthy individuals. Patients with BD showed significant normalization relative to the control group in connectivity between L insula and ACC and between R AMY to R Occipital pole. (C) Six-week changes (three points in time) of L insula-ACC connectivity in patients and healthy individuals. Patients with BD showed significant normalization relative to the control group in connectivity between L insula and ACC. ACC = anterior cingulate cortex; HC = healthy controls; L = left; Li = lithium; OFC = orbital frontal cortex; Que = quetiapine; R = right; WK = week; VLPFC = ventrolateral prefrontal cortex.
For the model testing for change between baseline and week 6, we did not find significant differences in connectivity changes between the lithium and quetiapine groups. Therefore, the combined treatment group was used for further analyses. The group-by-time interaction effect was significant for functional connectivity between left insula and bilateral ACC (F = 4.79, p = 0.031) and right amygdala and right occipital pole (F = 5.18, p = 0.025) (Figure 2B). For the model including all three points in time, we did not find significant differences in change of connectivity over the study among the lithium, quetiapine, and healthy groups. Combining the lithium and quetiapine groups for further analyses, we found that the group-by-time interaction effect was significant only in the connectivity between left insula and bilateral ACC (F = 3.74, p = 0.026) (Figure 2C). For within group longitudinal analysis of patients, we found connectivity between left VLPFC and left temporal pole (F = 6.02, p = 0.003), left OFC and right postcentral gyrus (F = 6.66, p = 0.002) and left insula and bilateral ACC (F = 7.20, p = 0.001) decreased significantly towards healthy individuals over time in the patient group. Connectivity did not change in healthy individuals for any seed region.
Since the included participants had different pubertal development (Tanner Stage II, n = 4; Tanner stage III, n = 26; Tanner stage IV, n = 46; Tanner stage V, n = 50), in exploratory analyses we tested for differences in in connectivity change over the course of treatment in relation to Tanner stage of patients, combining Tanner II and III stage participants in this analysis. We did not find significant interactions between group, time and Tanner stage in any analysis.
Correlation Analysis
Since one-week connectivity changes were only seen in the quetiapine treatment group, correlational analyses focused on the two treatment groups separately. The six-week changes were explored using the combined patient group. Change in connectivity at 1 week was not correlated with changes in manic symptom severity at one week (p > 0.05) or cognitive task performance in either the lithium or quetiapine treatment group.
Correlation analysis with the combined patient group testing for relations between connectivity and symptom change at six weeks demonstrated that connectivity between left VLPFC and left temporal pole was associated with changes in YMRS scores (r = 0.27, p = 0.029, Figure 3A). Moreover, baseline connectivity between left insula and bilateral ACC predicted change in YMRS scores at 6 weeks (r = 0.24, p = 0.047, Figure 3B).
Figure 3. Correlations Between Young Mania Rating Scale (YMRS) Changes and Baseline Functional Connectivity Alterations.
Note: (A) L VLPFC – L temporal pole connectivity changes were positively correlated with YMRS changes (baseline – week six, %). (B) baseline L insula and ACC connectivity was positively correlated with YMRS changes (baseline – week six, %). YMRS, the Young Mania Rating Scale; WK, week; ACC = anterior cingulate cortex; L = left; R = right; VLPFC = ventrolateral prefrontal cortex.
We followed these analyses with examination of characteristics of patients with the greatest change in symptoms and connectivity between left VLPFC and left temporal pole during treatment. We identified the patients with greater-than-average change in both connectivity and YMRS scores, which included 23 of the 71 patients. We compared the age, sex and YMRS scores between this subgroup and the remaining patients, and found that the patients with greater symptom and connectivity changes were older (15.08 ± 1.75 years old vs 14.04 ± 1.96 years old, t = 2.250, p = 0.029), but did not differ in demographic or baseline clinical features (p > 0.05).
Considering the possibility that the connectivity changes precede the changes in symptoms, we looked at correlation between change in connectivity between baseline and week 1 and the change in YMRS between baseline and week 6. While, we did not find any significant correlation results (p > 0.05).
Discussion
In the current study, we examined brain connectivity abnormalities and pharmaceutical treatment effects on these deficits in youth with mixed/manic states of BD. Case control differences at baseline were observed in regions related to sensory processing, affective reactivity and cognitive control. Some of these functional connectivity deficits were reduced by treatment with lithium and quetiapine within six weeks. Normalization of connectivity was evident at 1-week with quetiapine but not lithium therapy.
At baseline, individuals with BD displayed decreased connectivity between bilateral ACC with left insula and left VLPFC. Connectivity between insula and ACC is an important aspect of the salience network and autonomic reactivity.35 Via this network, the exteroceptive and interoceptive stimuli can be used to allocate attentional resources to context appropriate aspects of the environment.36 The salience network abnormalities have been widely reported by fMRI studies in BD.37 Consistent with our results, McTeague et al. found that the salience network disruption was related to dysfunction of emotional processing in a variety of psychiatric disorders, including BD.38 In correlation analyses, our results showed that the baseline connectivity between insula and ACC was correlated with treatment-related reduction of YMRS ratings, suggesting that salience network dysfunction provide a predictor of treatment response in youth with BD.
Atypical connectivity between VLPFC and ACC was also identified in patients prior to treatment. Increased emotional reactivity of amygdala has been reported in prior studies,12,39 and the VLPFC and rostral ACC are involved in down-regulation of amygdala reactivity.40 As a result, reduced connectivity between VLPFC and ACC might be associated with hyperactivity of the amygdala that has been previously reported in BD.
We also observed increased connectivity between our seeds and temporal pole, occipital pole, and right postcentral gyrus. The temporal and occipital poles and the postcentral gyrus are important areas for affective, visual and sensorimotor processes, respectively systems. Prior studies shown that the activity in the interoceptive system (high physiological arousal states) are correlated with heightened activity in exteroceptive sensory systems41, perhaps related to shared connectivity.42 Our observation of increased connectivity between our seeds related to emotion processing (VLPFC, OFC and amygdala) and sensory cortex without an explicit emotional stimulus may indicate a heightened reactivity in both systems in the context of increased cognitive demand. This pattern of results is consistent with our first hypothesis that abnormal connectivity related to emotion and attention in patients with BD are prominent in BD and can be reduced with medications that are clinically effective in treating mania symptoms.
Beyond informative observations of baseline alterations in the functional brain connectome in manic bipolar youth prior to treatment, our analyses demonstrate that clinically effective psychopharmacological treatment can reduce functional brain connectivity alterations evident during acute mixed/manic episodes.43,44 After the first week of treatment, patients treated with quetiapine, but not lithium, showed a significant shift toward normalized functional connectivity between left VLPFC and left temporal pole, and between left OFC and right postcentral gyrus. We did not find significant correlations between change in connectivity and mania symptoms at one-week follow-up, suggesting that the improvement in connectivity and symptom severity may follow somewhat different courses in different patients. Future work is needed with more frequent assessment of early changes in brain function and symptom severity to better understand these relationships.
After six-weeks of treatment, no significant differences between the lithium and quetiapine group were found, though a normalization of connectivity in the combined group of treated patients with BD. This suggests overlaps in the broad systems-level changes in the functional brain connectome after treatment with lithium and quetiapine, something reported in prior fMRI studies. Dandash et al. found that lithium led to more rapid treatment normalization of abnormally increased connectivity between ventral striatum and cerebellum22 during a three-month treatment period when compared with quetiapine. Using a sustained attentional task with emotional stimuli, Strakowski et al. found that lithium treatment showed more rapid suppression of amygdala overactivation in BD compared with quetiapine treatment after one-week of treatment.45 Differences in observed differential drug effects on brain connectivity between ours and these studies need to be considered in the context of several significant differences in study design and methodology. First, both Dandash et al. and Strakowski et al. included adults diagnosed with bipolar I disorder, while we recruited youth. Second, BD patients were receiving different treatments at baseline, as Dandash et al. study recruited patients that were receiving a combination of quetiapine and lithium and then randomized to monotherapy with one of these treatments after remission. Third, the fMRI task design and ROI selection were different. Dandash et al. treated ventral and dorsal areas of the caudate nucleus and putamen as ROIs, and Strakowski et al. used an attention task with emotion distractors with a focus on prefrontal regions related to emotion processing as their ROIs. In the current study, we used a simple attention/working memory task with no emotional features in task stimuli. Fourth, fMRI measures were different. Dandash et al. and our study used FC as the target measurement, while Strakowski et al. used regional ROI activation as the target measurement. Therefore, it is difficult to establish mechanisms of different treatment outcomes, which notably were in speed of functional connectivity changes rather than in varying brain regions where treatments exerted effects. This pattern suggests both drugs impact functional connectivity alterations in overlapping ways at the level of the functional brain connectome. Future studies with larger samples, considering dose-related effects influencing speed of clinical recovery, and with more frequent imaging and behavioral ratings in the early treatment period, may help establish the validity of our findings and better clarify their relation to distinct and overlapping areas of treatment effect on brain function.
We also observed that task performance was not improved across the course of treatment, indicating that recovery of function seen in brain connectivity and mania symptoms was not evident in the cognitive domain, at least with this task. This observation is consistent with prior studies that reported CPT and other cognitive impairments in bipolar disorder and in studies of cognition across studies of other affective disorders and psychotic illnesses.4,46–48 Future fMRI studies including a wider range of task conditions may better clarify the stability of cognitive deficits seen during acute episodes of illness, and relationships changes in manic symptoms, brain function and cognitive functions over time.
The present study had several limitations that merit consideration. First, we used multiple seeds to evaluate functional connectivity, which reduces statistical power and limits assessment of connectivity changes involving other brain regions. While all seeds were chosen based on a priori knowledge of brain regions believed to be affected in BD, other interregional connectivity features may be important for fully understanding mechanisms of treatment effect and differential effects of treatments on brain systems.34 Second, the current study focused on short-term changes in functional connectivity influenced by medications. Future efforts to clarify the long-term effects of medication effects on functional connectivity in patients with BD are needed. Third, our study focused on a cognitive task with its own advantages and limitations, and the interplay between attention and emotion processing systems might be better clarified using other task conditions.
Despite these limitations, the present study is one of few fMRI studies focusing on the changes in functional connectivity during cognitive task performance related to effective pharmacological treatment in youth with mixed/manic BD. Significant normalization of some alterations of functional brain connectivity were observed as early as the first week of treatment with quetiapine, suggesting that imaging approaches as used in our study may provide a useful approach for establishing target engagement for standard as well as promising novel treatments for BD.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Mental Health (NIMH; Grant No. 5R01MH080973 DelBello) and the National Natural Science Foundation (Grant Nos. 81621003 QYG and 81820108018 QYG/JAS). The study funders had no role in the design or reporting of the study.
The research was performed with permission from the University of Cincinnati and Children’s Hospital Medical Center Institutional Review Boards.
The authors would like to thank the children and families who participated in this study for their dedication and contribution to the research community.
Disclosure:
Dr. Patino has received research support from Acadia, Allergan, Alkermes, Janssen, Johnson and Johnson, Lundbeck, Otsuka, Pfizer, Sunovion, and Supernus. Prof. Strawn has received research support from the National Institutes of Health, AbbVie, Neuronetics, and Otsuka. He has received material support from and provided consultation to Myriad Genetics and royalties from the publication of two texts (Springer) and has served as an author for UpToDate and an Associate Editor for Current Psychiatry. He has spoken in CME presentations for the Neuroscience Education Institute, MedScape, the American Academy of Child & Adolescent Psychiatry, the American Academy of Pediatrics, and CMEology. He has provided consultation to the Food and Drug Administration and Intracellular Therapeutics. Prof. Sweeney has served as a consultant to VeriSci. Prof. Adler has served on the lecture bureaus for Otsuka and Janssen. He has received research support from Merck, Forest, and Alkermes and has provided consultation for Janssen. Prof. DelBello has served on the lecture bureau for Otsuka. She has received research support from Acadia, Allergan, Alkermes, Janssen, Johnson and Johnson, Lundbeck, Otsuka, Pfizer, Sunovion, and Supernus and has provided consultation or advisory board services for Alkermes, Allergan, Assurex, CMEology, Janssen, Johnson and Johnson, Lundbeck, Neuronetics, Otsuka, Pfizer, Sunovion, and Supernus. Drs. Li, Lei, Welge, Fleck, Profs. Gong and Strakowski, Mr. Tallman, Ms. Ai, Mr. Blom, and Ms. Klein have reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Clinical trial registration information: Multimodal Neuroimaging of Treatment Effects in Adolescent Mania; https://clinicaltrials.gov/; NCT00893581.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Wenbin Li, West China Hospital of Sichuan University, Sichuan, China.; Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio. The First Affiliated Hospital of Zhengzhou, University, Zhengzhou, Henan, China.
Du Lei, Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio..
Messrs. Maxwell J. Tallman, Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio..
Ms. Yuan Ai, West China Hospital of Sichuan University, Sichuan, China.; Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio.
Jeffrey A. Welge, Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio..
Thomas J. Blom, Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio..
David E. Fleck, Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio..
Christina C. Klein, Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio..
Luis R. Patino, Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio..
Profs. Jeffrey R. Strawn, Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio..
Profs. Qiyong Gong, West China Hospital of Sichuan University, Sichuan, China.; Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu, Sichuan, China.
Prof. Stephen M. Strakowski, Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio.; Dell Medical School, University of Texas at Austin, Texas.
John A. Sweeney, West China Hospital of Sichuan University, Sichuan, China.; Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio.
Caleb M. Adler, Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio..
Melissa P. DelBello, Division of Bipolar Disorders Research, University of Cincinnati College of Medicine, Ohio..
References:
- 1.Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry. 2002;59(2):115–123. doi: 10.1001/archpsyc.59.2.115 [DOI] [PubMed] [Google Scholar]
- 2.Angst J. The emerging epidemiology of hypomania and bipolar II disorder. J Affect Disord. 1998;50(2–3):143–151. doi: 10.1016/s0165-0327(98)00142-6 [DOI] [PubMed] [Google Scholar]
- 3.Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. Oct 1 2000;48(7):674–84. doi: 10.1016/s0006-3223(00)00910-0 [DOI] [PubMed] [Google Scholar]
- 4.Pavuluri MN, West A, Hill SK, Jindal K, Sweeney JA Neurocognitive function in pediatric bipolar disorder: 3-year follow-up shows cognitive development lagging behind healthy youths. J Am Acad Child Adolesc Psychiatry. Mar 2009;48(3):299–307. doi: 10.1097/CHI.0b013e318196b907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 2012;14(4):326–339. doi: 10.1111/j.1399-5618.2012.01021.x [DOI] [PubMed] [Google Scholar]
- 6.Passarotti AM, Sweeney JA, Pavuluri MN. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. J Int Neuropsychol Soc. Jan 2010;16(1):106–17. doi: 10.1017/s1355617709991019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavuluri MN, Passarotti AM, Mohammed T, Carbray JA, Sweeney JA. Enhanced working and verbal memory after lamotrigine treatment in pediatric bipolar disorder. Bipolar Disord. Mar 2010;12(2):213–20. doi: 10.1111/j.1399-5618.2010.00792.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleck DE, Eliassen JC, Durling M, et al. Functional MRI of sustained attention in bipolar mania. Mol Psychiatry. 2012;17(3):325–336. doi: 10.1038/mp.2010.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adler CM, Delbello MP, Mills NP, Schmithorst V, Holland S, Strakowski SM. Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disord. Dec 2005;7(6):577–88. doi: 10.1111/j.1399-5618.2005.00257.x [DOI] [PubMed] [Google Scholar]
- 10.Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. Sep 2005;162(9):1697–705. doi: 10.1176/appi.ajp.162.9.1697 [DOI] [PubMed] [Google Scholar]
- 11.Lee M-S, Anumagalla P, Talluri P, Pavuluri MN. Attentional engagement increases inferior frontal gyrus activity and mutes limbic activity in pediatric bipolar disorder: Meta-analyses of fMRI studies. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:14–19. doi: 10.1016/j.pnpbp.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 12.Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. Apr 15 2008;162(3):244–55. doi: 10.1016/j.pscychresns.2007.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. Oct 2010;49(10):1064–80. doi: 10.1016/j.jaac.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang KD. Course and impact of bipolar disorder in young patients. J Clin Psychiatry. Feb 2010;71(2):e05. doi: 10.4088/JCP.8125tx7c [DOI] [PubMed] [Google Scholar]
- 15.Rey G, Piguet C, Benders A, et al. Resting-state functional connectivity of emotion regulation networks in euthymic and non-euthymic bipolar disorder patients. Eur Psychiatry. Apr 2016;34:56–63. doi: 10.1016/j.eurpsy.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Wang Y, Huang H, et al. Abnormal dynamic functional network connectivity in unmedicated bipolar and major depressive disorders based on the triple-network model. Psychol Med. Feb 2020;50(3):465–474. doi: 10.1017/s003329171900028x [DOI] [PubMed] [Google Scholar]
- 17.Delbello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. Mar 2006;45(3):305–313. doi: 10.1097/01.chi.0000194567.63289.97 [DOI] [PubMed] [Google Scholar]
- 18.Delbello MP, Schwiers ML, Rosenberg HL, Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. Oct 2002;41(10):1216–23. doi: 10.1097/00004583-200210000-00011 [DOI] [PubMed] [Google Scholar]
- 19.Patino LR, Klein CC, Strawn JR, et al. A Randomized, Double-Blind, Controlled Trial of Lithium Versus Quetiapine for the Treatment of Acute Mania in Youth with Early Course Bipolar Disorder. J Child Adolesc Psychopharmacol. Sep 14 2021;doi: 10.1089/cap.2021.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei D, Li W, Tallman MJ, et al. Changes in the brain structural connectome after a prospective randomized clinical trial of lithium and quetiapine treatment in youth with bipolar disorder. Neuropsychopharmacology. Jun 2021;46(7):1315–1323. doi: 10.1038/s41386-021-00989-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Xiao Y, Sun H, et al. Discrete patterns of cortical thickness in youth with bipolar disorder differentially predict treatment response to quetiapine but not lithium. Neuropsychopharmacology. Oct 2018;43(11):2256–2263. doi: 10.1038/s41386-018-0120-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dandash O, Yücel M, Daglas R, et al. Differential effect of quetiapine and lithium on functional connectivity of the striatum in first episode mania. Translational psychiatry. 2018;8(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavuluri MN, Passarotti AM, Fitzgerald JM, Wegbreit E, Sweeney JA. Risperidone and divalproex differentially engage the fronto-striato-temporal circuitry in pediatric mania: a pharmacological functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. Feb 2012;51(2):157–170.e5. doi: 10.1016/j.jaac.2011.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavuluri MN, Sweeney JA. Integrating functional brain neuroimaging and developmental cognitive neuroscience in child psychiatry research. J Am Acad Child Adolesc Psychiatry. Nov 2008;47(11):1273–88. doi: 10.1097/CHI.0b013e318185d2d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. Sep-Oct 2004;75(5):1357–72. doi: 10.1111/j.1467-8624.2004.00745.x [DOI] [PubMed] [Google Scholar]
- 26.Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary FMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29(9):1734. [DOI] [PubMed] [Google Scholar]
- 27.Geller B, Zimerman B, Williams M, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40(4):450–455. doi: 10.1097/00004583-200104000-00014 [DOI] [PubMed] [Google Scholar]
- 28.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429 [DOI] [PubMed] [Google Scholar]
- 29.Poznanski EO, Mokros HB. Children’s depression rating scale, revised (CDRS-R). Western Psychological Services; Los Angeles; 1996. [Google Scholar]
- 30.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010 [DOI] [PubMed] [Google Scholar]
- 31.Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66(6):918–920. [PubMed] [Google Scholar]
- 32.Stano JF. Wechsler abbreviated scale of intelligence. Rehabilitation Counseling Bulletin. 2004;48(1):56. [Google Scholar]
- 33.Wegbreit E, Ellis JA, Nandam A, et al. Amygdala functional connectivity predicts pharmacotherapy outcome in pediatric bipolar disorder. Brain Connect. 2011;1(5):411–22. doi: 10.1089/brain.2011.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor JG, Fragopanagos NF. The interaction of attention and emotion. Neural networks : the official journal of the International Neural Network Society. 2005;18(4):353–369. doi: 10.1016/j.neunet.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 35.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci. Jul 1998;10(4):525–35. doi: 10.1162/089892998562924 [DOI] [PubMed] [Google Scholar]
- 37.Magioncalda P, Martino M, Conio B, et al. Functional connectivity and neuronal variability of resting state activity in bipolar disorder--reduction and decoupling in anterior cortical midline structures. Hum Brain Mapp. 2015;36(2):666–682. doi: 10.1002/hbm.22655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McTeague LM, Rosenberg BM, Lopez JW, et al. Identification of Common Neural Circuit Disruptions in Emotional Processing Across Psychiatric Disorders. The American journal of psychiatry. 2020:appiajp201918111271-appiajp201918111271. doi: 10.1176/appi.ajp.2019.18111271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(3):308–319. doi: 10.1097/CHI.0b013e3181948fc7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry A, Roberts G, Mitchell PB, Breakspear M. Connectomics of bipolar disorder: a critical review, and evidence for dynamic instabilities within interoceptive networks. Mol Psychiatry. Sep 2019;24(9):1296–1318. doi: 10.1038/s41380-018-0267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seth AK, Friston KJ. Active interoceptive inference and the emotional brain. Philos Trans R Soc Lond B Biol Sci. Nov 19 2016;371(1708)doi: 10.1098/rstb.2016.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerullo MA, Fleck DE, Eliassen JC, et al. A longitudinal functional connectivity analysis of the amygdala in bipolar I disorder across mood states. Bipolar Disord. Mar 2012;14(2):175–84. doi: 10.1111/j.1399-5618.2012.01002.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altinay M, Karne H, Anand A. Lithium monotherapy associated clinical improvement effects on amygdala-ventromedial prefrontal cortex resting state connectivity in bipolar disorder. J Affect Disord. Jan 1 2018;225:4–12. doi: 10.1016/j.jad.2017.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strakowski SM, Fleck DE, Welge J, et al. fMRI brain activation changes following treatment of a first bipolar manic episode. Bipolar Disord. Sep 2016;18(6):490–501. doi: 10.1111/bdi.12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strakowski SM, Fleck DE, DelBello MP, et al. Characterizing impulsivity in mania. Bipolar Disord. Feb 2009;11(1):41–51. doi: 10.1111/j.1399-5618.2008.00658.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilder-Willis KE, Sax KW, Rosenberg HL, Fleck DE, Shear PK, Strakowski SM. Persistent attentional dysfunction in remitted bipolar disorder. Bipolar Disord. Apr 2001;3(2):58–62. doi: 10.1034/j.1399-5618.2001.030202.x [DOI] [PubMed] [Google Scholar]
- 48.Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naïve patients with schizophrenia. Schizophr Res. May 1 2004;68(1):49–63. doi: 10.1016/s0920-9964(03)00213-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.