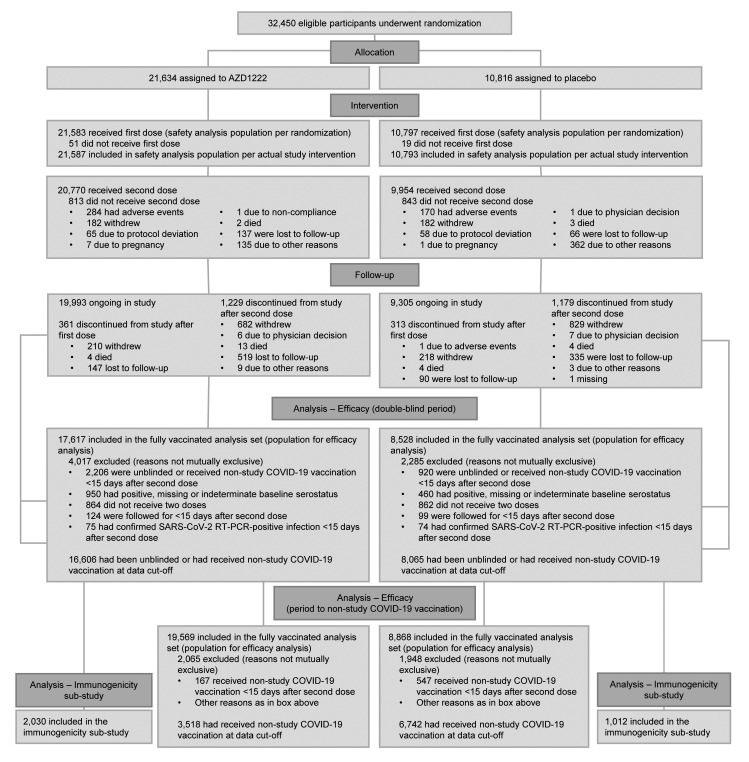
Figure 1. Participant disposition during trial.
Overall number randomized and number randomized to AZD1222 are 1 lower than in the primary analysis (8) due to identification of double-counting of 1 participant. In the placebo arm, 1 participant was not included in the primary analysis due to record deactivation, but has been reinstated at this analysis. FVAS population numbers differ from those in the primary analysis because, with additional follow up, additional participants achieved the milestone of 15 days after the second dose and became eligible for these populations, but also some participants were excluded from these populations based on newly obtained information regarding prior infections, baseline serology and receipt of nonstudy COVID-19 vaccinations.