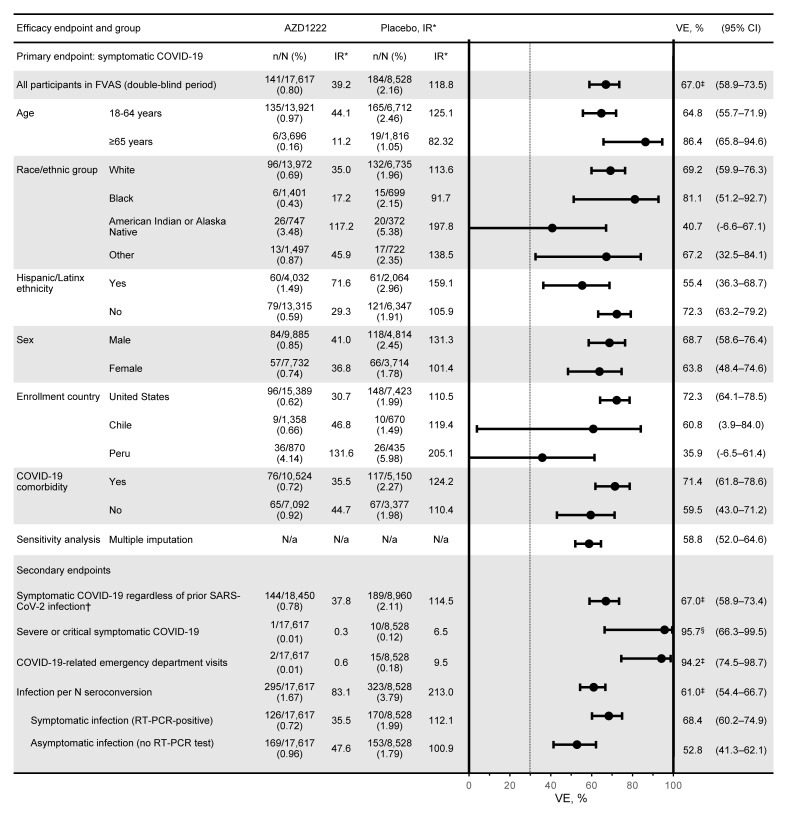
Figure 2. Forest plot of estimated efficacy of AZD1222 versus placebo in the double-blind period.
Plot shows estimated AZD1222 efficacy 15 or more days after the second dose for the primary and secondary efficacy end points in the FVAS population for the double-blind period, with censoring for unblinding or nonstudy COVID-19 vaccination (AZD1222, n = 17,617; placebo, n = 8528). Total follow-up was 3.60 and 1.55 × 1000 person years in the AZD1222 and placebo groups. The dotted vertical line represents the nominally statistically significant criterion of a lower CI greater than 30% applicable to the primary end point and is shown for reference. VE was calculated as (1 minus relative risk) × 100, with relative risk estimated using Poisson’s regression model with robust variance adjusted for follow-up time and with trial group and age group (18–64 versus ≥65 years) as covariates. *Per 1000 person years. †The FVAS includes all participants who were SARS-CoV-2 seronegative at baseline; this population (n = 18,450 in AZD1222 group; n = 8960 in placebo group) includes participants regardless of prior SARS-CoV-2 infection. ‡P < 0.001; §P = 0.03. IR, incidence rate.