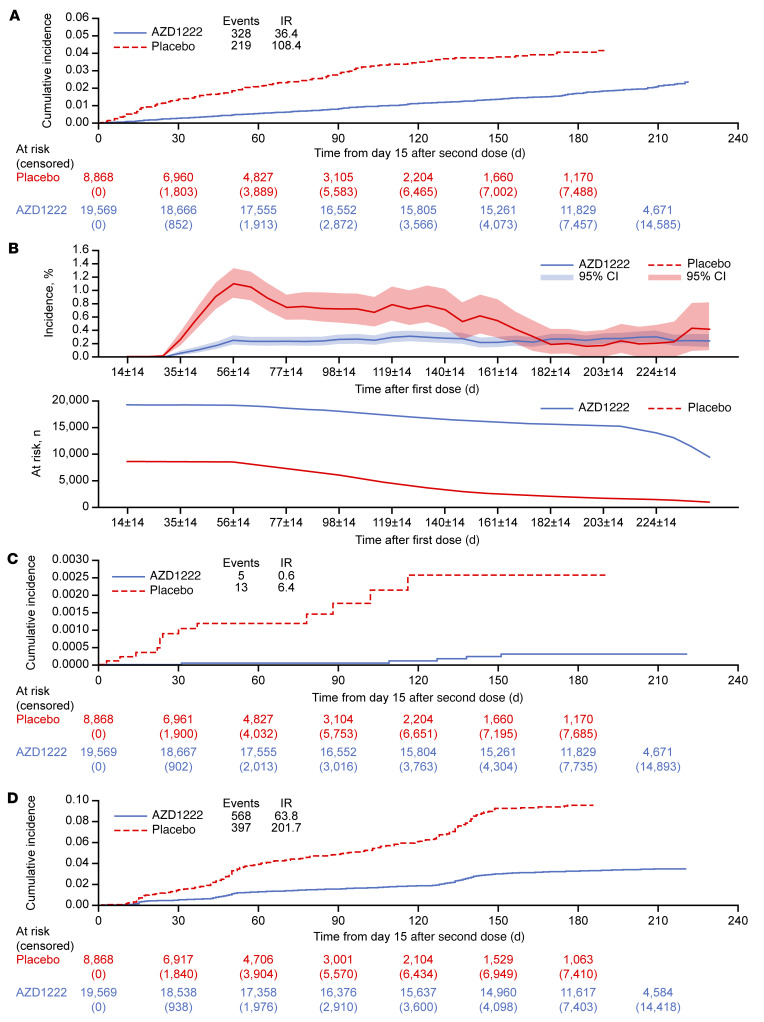
Figure 3. Estimated efficacy of AZD1222 versus placebo for the prevention of COVID-19 and SARS-CoV-2 infection during the period to nonstudy COVID-19 vaccination.
(A) Cumulative incidence of SARS-CoV-2 RT-PCR–positive symptomatic illness occurring 15 or more days after the second dose (time 0 = day 15 after the second dose) in the FVAS population for the period to nonstudy COVID-19 vaccination (AZD1222, n = 19,569; placebo, n = 8868), with censoring for nonstudy COVID-19 vaccination, regardless of unblinding. (B) Incidence of SARS-CoV-2 RT-PCR–positive symptomatic illness events and decrease in the at-risk population over time from first dose during the period to nonstudy COVID-19 vaccination. The at-risk population curves show the numbers of participants in the FVAS who have not been censored and were available for analysis at the corresponding time point. Cumulative incidence of (C) severe or critical symptomatic COVID-19 and (D) SARS-CoV-2 infection, as defined by seroconversion rate from negative at baseline to positive for SARS-CoV-2 nucleocapsid antibody at 15 or more days after the second dose, regardless of symptoms, in the FVAS population for the period to nonstudy COVID-19 vaccination (AZD1222, n = 19,569; placebo, n = 8868), with censoring for nonstudy COVID-19 vaccination, regardless of unblinding. For panels A, C, and D, time to first event was from time of second dose administration, calculated as follows: (date of SARS-CoV-2-positive test) – (date of second dose of AZD1222 or placebo + 14 days) + 1. For censored participants, censoring time was from date of second dose of AZD1222 or placebo plus 14 days to the last time observed before data cutoff (July 30, 2021). Cumulative incidences were estimated using the Kaplan-Meier method. Cumulative incidence curves were truncated at the point at which less than 10% of the starting population remained at risk. IR, incidence rate per 1000 person years.