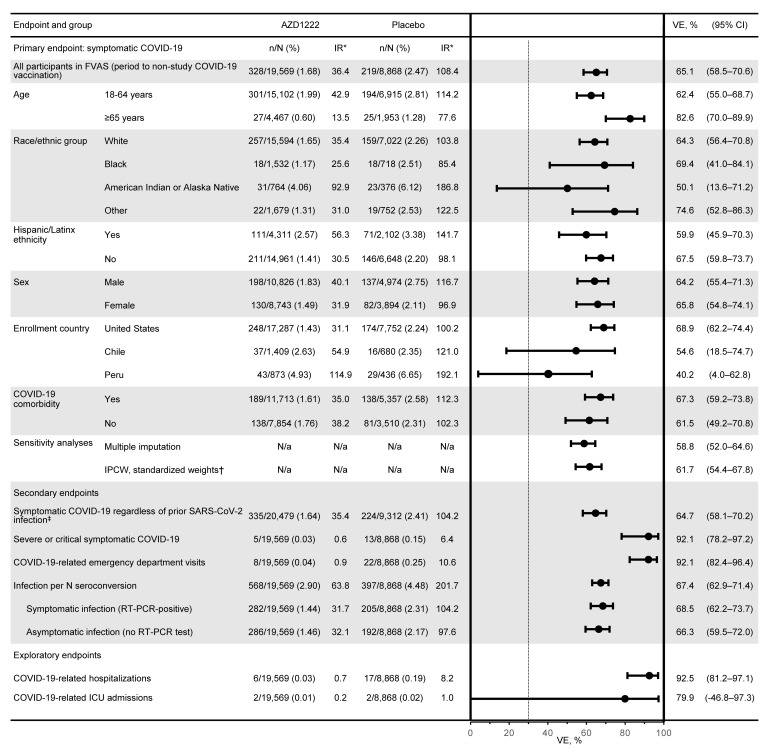
Figure 4. Forest plot of efficacy estimates of AZD1222 versus placebo in the period up to receipt of nonstudy COVID-19 vaccination.
Plot shows estimated AZD1222 efficacy 15 or more days after the second dose for the primary, secondary, and exploratory efficacy end points in the FVAS population for the period to nonstudy COVID-19 vaccination, with censoring for nonstudy COVID-19 vaccination, regardless of unblinding (AZD1222, n = 19,569; placebo, n = 8868). Total follow-up was 9.01 and 2.02 × 1000 person years in the AZD1222 and placebo groups. The dotted vertical line represents the nominally statistically significant criterion of a lower CI greater than 30% applicable to the primary end point and is shown for reference. VE was calculated as (1 minus relative risk) × 100, with relative risk estimated using Poisson’s regression model with robust variance adjusted for follow-up time and with trial group and age group (18–64 versus ≥65 years) as covariates. *Per 1000 person-years. †Results from an IPCW method applied to right censoring and using standardized weights. See Statistics section of Methods for methodology and Supplemental Table 11 for additional analyses and information. ‡The FVAS includes all participants who were SARS-CoV-2 seronegative at baseline; this population (n = 20,479 in AZD1222 group; n = 9312 in placebo group) includes participants regardless of prior SARS-CoV-2 infection.