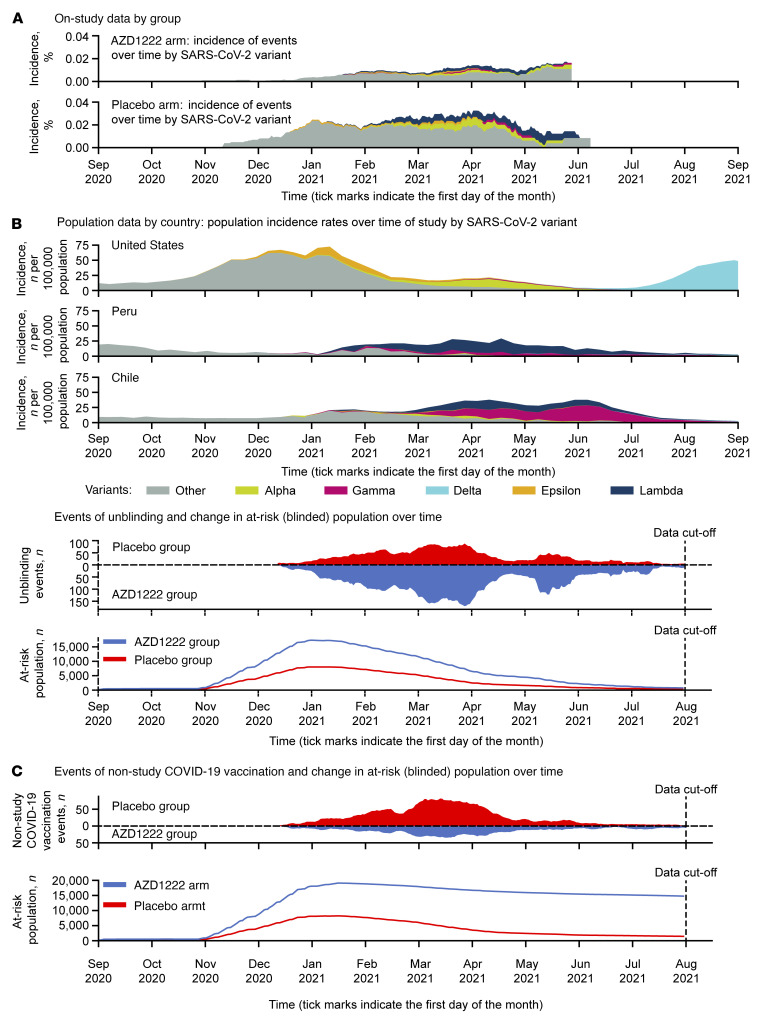
Figure 6. Incidence of SARS-CoV-2 variants, numbers at risk, and unblinding/receipt of nonstudy COVID-19 vaccination over the time course of the trial.
(A) Incidence of variants observed in cases of RT-PCR–confirmed SARS-CoV-2 infection in the placebo and AZD1222 arms of the trial, truncated at the point at which less than 10% of the starting population remained at risk, and incidence of confirmed cases in population data from the US, Peru, and Chile during the time of study (data derived from COVID-19 Data Repository by the Center for Systems Science and Engineering [CSSE] at Johns Hopkins University, ref. 30, available at https://github.com/owid/covid-19-data/tree/71b0337018fe20d469aa9014e3a8003d900a2b5b, commit ID: 71b0337018fe20d469aa9014e3a8003d900a2b5b; and from GISAID, EPI_SET_220825fk, https://doi.org/10.55876/gis8.220825fk), along with timing of participants being on study, with censoring for (B) unblinding or nonstudy COVID-19 vaccination, or (C) nonstudy COVID-19 vaccination only in the FVAS populations for these 2 analyses.