Abstract
Introduction
Clematis simensis is one of the most widely used medicinal plant for the treatment of wound traditionally. However, its claim was not scientifically tested, the current study therefore assessed the anti-inflammatory and wound healing properties of 80% methanol leaf extract of C. simensis.
Methods
The dried and powdered leaf of C. simensis was macerated with 80% methanol. The topical ointment was then made in two concentrations (5% and 10% w/w), and two models, excision and incision, were used to test the extract’s capacity to treat wounds in mice. In addition, anti-inflammatory test was also conducted using carrageenan-induced hind paw edema model in three doses (100, 200, and 400 mg/kg) in rats. The DPPH test was used to determine the extract’s anti-oxidant properties where ascorbic acid was used as standard agent.
Results
When wounds were treated with ointments containing 5% and 10% (w/w) extract, the rate of wound contraction, the length of time it took for the epithelium to form, and the strength of the skin to break were all significantly increased (p < 0.05). There was no discernible difference in wound healing activity between the 10% (w/w) and 5% (w/w) extracts. Moreover, they had also similar impact as that of the positive control nitrofurazone in wound healing activity. Compared to the negative control, C. simensis extract considerably (p < 0.01-p < 0.001) reduced inflammation. The extract also demonstrated antioxidant activity with IC50 values of 0.7 mg/mL for the extract and 1.04 mg/mL for ascorbic acid, respectively.
Conclusion
Overall, it is plausible to draw the conclusion that C. simensis 80% methanol extract possesses wound healing activity, perhaps as a result of its anti-inflammatory and antioxidant effects.
Keywords: wound healing, anti-inflammatory, excision, incision, Clematis simensis
Introduction
Skin is the major component of the integumentary system which serves as a barrier against the external environment as well as serves as a major homeostasis organ for temperature and vitamin-D regulation.1 When there is injury or damage to the anatomical structure of skin, it creates wound. Wound is a physical, chemical, thermal, microbiological, or immunological disturbance in the structural, cellular, and functional epithelial integrity of the skin.2
Wounds can be caused by a number of events, including surgery, traumas, extrinsic forces (such as pressure, burns, and cuts), or pathologic disorders such as diabetes or vascular disease.3 Depending on the underlying causes and outcomes, these types of injury are characterized as acute or chronic wounds. Acute wounds normally heal in a systematic and suitable manner, culminating in the long-term restoration of anatomical and functional integrity. Chronic wounds, on the other hand, are unable to acquire optimal anatomical and functional integrity.4
Wound repair mechanism entails the involvement of various inflammatory, immune and vasoactive mediators.5 The linear nature of the repair process results in the growth factors driving cell proliferation, which integrates dynamic changes involving soluble mediators, blood cells, extracellular matrix synthesis, and proliferation of parenchymal cells. Inflammatory response, cell proliferation, and the synthesis of the components that make up the extracellular matrix are the three stages of repair that occur as a result of cellular and biochemical events in wound healing culminated by remodeling.2
Wound is treated by debridement, irrigation, antibiotics, tissue grafts, and proteolytic enzyme methods, which have undesirable side effects.6 Because chronic wounds might house bacteria, topical preparations of the following antibiotics (amikacin, bacitracin, chloramphenicol, clindamycin, gentamicin, nitrofurazone, and polymyxin B) are chosen based on their capacity to suppress the growth of pathogenic organisms.7 In addition, anti-inflammatory medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are also used to halt the inflammatory sequel of wound.8 When these medications are used as a topical preparation at the early stage of wound there is reduced risk of systemic side effects, and have higher concentration of the drug in the affected area as well as provides the advantage of quantified drug usage.9
Despite the efficacy of the aforementioned conventional medications, they are not free from limitations such as bacterial resistance, adverse effects, costs as well as improper use of these medicines.7,9 Moreover, clinical audits have found that less than 4% of the medications in Western pharmacopoeias are indicated for the treatment of wounds, and their effectiveness for promoting rapid wound healing is unsatisfactory.10
Thus, finding an alternate treatment that is both safe and effective is crucial. Herbs have served as the primary starting point for the discovery of new medications from the limited sources available.3 Several studies in Ethiopia show that various medicinal plants have wound healing property. Plants like Achyranthes aspera,11 Allophylus abyssinicus,12 Kalanchoe petitiana A. Rich,13 Rumexa abyssinicus,14 Vernonia auriculifera Hiern,15 Zehneria scabra16 and many other are scientifically proved to exhibit wound healing activity.
Despite the fact that C. simensis, the experimental plant, has historically been used to promote wound healing, to our knowledge there are no studies to support this claim.17 Thus, the purpose of this study was to assess how well the leaf extract of C. simensis helped with in-vivo wound healing and anti-inflammatory effect.
Materials and Methods
Chemicals, Drugs and Animals
The following chemicals and reagents were used; Aspirin (Bayer AG, Germany), carrageenan (Sigma-Aldrich Steinheim, Germany), cetostearyl alcohol, wool fat, hydrochloric acid, sulphuric acid, hard paraffin, white soft paraffin (BDH Laboratory Supplies Poole, England), ketamine hydrochloride (Rotex Medica, Germany), methanol (Blulux, India, purchased from ZAF pharmaceuticals Pvt.Ltd.Co), nitrofurazone 0.2% (Shanghai General Pharmaceuticals Co, Ltd, China).
Plant Material
C. simensis leaves were obtained from the Gulele Botanical Garden in the Gulele sub-city of Addis Ababa, Ethiopia, in the month of February 2022. Mr. Melaku Wondafrash performed botanical identification and authentication at the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University, and a voucher specimen (number NT-001) was placed for future reference.
Experimental Animals
The animal house of the College of Health Sciences’ School of Pharmacy provided 45 adult Swiss albino mice of both sex that weighed 25–30 g and were 6–8 weeks old (animals used for excision and incision wound models). Twenty-five Wistar albino rats (Rattus norvegicus), weighing 250–350g and aged 4–6 months, were obtained from the Ethiopian Public Health Institute (EPHI) (animals used for anti-inflammatory activity test). The animals were given unlimited access to food and water as well as a 12-hour cycle of light and darkness were maintained. Before the trial started, the animals were allowed a week to get used to the laboratory setting. The appropriate protocols18 for the handling and use of experimental animals were followed during every procedure. The Ethical Review Committee of Addis Ababa University, College of Health Sciences, School of Pharmacy (ERB/SOP/474/14/2022) granted its approval to the experimental procedure.
Plant Extraction
Fresh leaves were gathered, cleaned under running water to remove debris and dust, and then dried for three weeks in the shade. The leaves were roughly ground using a grinder. The powdered substance (340 g) was macerated in a conical flask for three days at solute to solvent ratio of 1:5 (w/v) with 5000 mL of 80% methanol. The mixture was vacuum-filtered through Whatman filter paper (No 1) after being filtered twice through a funnel with muslin cloth (Maidstone, UK). After filtration, the marc or residue was re-macerated three times to maximize yield. The filtrate was evaporated using a rotary evaporator (Buchii model R-200, Switzerland) set to 40°C in order to remove the methanol. Finally, a 37°C oven was used to solidify the concentrated aqueous solution. Until it was time to prepare the ointment, the extract was kept in the refrigerator.
Ointment Formulation
According to the British Pharmacopoeia (BP, 1988a), simple ointment was made using the following ingredients: wool fat (10 g), hard paraffin (10 g), cetostearyl alcohol (10 g), and white soft paraffin (170 g).19 The simple ointment base was created by melting the separate components in a beaker over a water bath in descending order of melting point while swirling constantly until they melted completely. After being removed from the water bath, the liquid was spun until it became absolutely cool.
The extract was ground in a mortar and pestle before being combined with other ingredients to create the 5% and 10% medicinal ointments. The 80% methanol extract was then combined with 95 g and 90 g of the ointment base, respectively, by mixing on the ointment slab, to create an ointment with a homogeneous consistency and a smooth texture. Finally, the extract ointment used in the experiment was transferred to a fresh container for topical use.
Phytochemical Screening
As indicated elsewhere,20 the extract was examined for its potential composition of several natural constituents, including alkaloids, flavonoids, phenols, steroids, tannins, terpenes, and saponins.
Acute Dermal Toxicity Test
The acute dermal toxicity OECD 404 guideline21 was adhered. Randomly chosen three female mice with normal skin surfaces were kept in independent cages. About 10% of the hair on the dorsal half of the body surface area was removed before the extract ointment was applied. The extract ointment (10%) was applied evenly over the entire shaved site for 24 hours in a thin layer. Gauze was applied to the area and held in place with non-irritating tape so it would stay in contact with the skin. After removing the gauze, the region was examined for irritation. The skin was observed for changes throughout the subsequent 14 days.
Grouping and Dosing of Animals
For the dermal toxicity and wound healing experiments, animals were randomly selected and housed singly in cages so that wound conditions would not be worsened by biting and the bedding materials. In the excision model, five mice per group were divided into four randomly chosen groups of mice. The following test and control ointments: simple ointment (negative control), 0.2% (w/v) nitrofurazone (positive control), and crude ointments of 5% (w/w) and 10% (w/w) were topically applied, respectively, to each of the groups. The incision wound model underwent the same procedures, with the addition of a fifth group, or the untreated group.
To assess the anti-inflammatory activity, five groups each comprised five rats were used in this model. Three different doses of the extract (100 mg/kg, 200 mg/kg, and 400 mg/kg) were given to groups I through V, respectively, along with the conventional medication aspirin (150 mg/kg). The dose was calculated using the plant product’s safe dose, which was established as above 2000 mg/kg via a limit test obtained from elsewhere.22 A maximum of 10 mL/kg of the full dosage was taken orally. In order to create an oral solution, distilled water was used to dissolve both the extract and aspirin.
Excision Wound Model
On the day of wounding, subcutaneous injections of ketamine (1 mL/kg) and diazepam (1 mL/kg) were used to anesthetize the animals. The animals’ dorsal fur was removed using a shaver, and the projected location of the wound was marked on the dorsal thoracic region of their backs, 1 cm from the vertebral column. Excision wounds were made by removing 300 mm2 of full-thickness skin from a particular area and leaving the wound out in the open.23 After that, the mice were separated into four groups of five each, and each animal was given its own cage, as was previously mentioned. It was decided that the day of the wounded would be Day 0. For the duration of the healing process, the groups received topical applications of the standard medication, extract, and simple ointment respectively. This model tracked the duration of epithelialization and wound contraction. Following wound development, wound contraction was measured as a percentage of contraction on alternate days.24
Measurement of Wound Contraction
Initially, the wound was traced onto a clear piece of paper to observe contractions, which promote in the first two weeks of wound healing. Graph paper with a millimeter scale was then used to make an impression.25 On days 0, 2, 4, 6, 8, 10, 12, 14, and 18 after the first injury, the wounds were observed, and the size of the wound was measured. The following formula was used to determine the wound healing impact, using the initial wound size of 300 mm2 as a baseline.23
% Wound contraction = Initial wound size – Specific day wound size x 100
Initial wound size
Epithelialization Time Measurement
The number of days needed for the scab to fall off without any remaining raw wound was taken into account while calculating the epithelialization duration.26
Incision Wound Model
Like the excision wound model, the animals underwent anesthesia. The dorsal fur of each mouse was shaved, and a three centimeter longitudinal paravertebral incision was made into the skin and subcutaneous tissue. The cut skin was then sewn together, one centimeter apart, with chromic catgut (2/0 metric-1/2 circle) and a curved needle. To make sure that all wound edges were correctly stitched together, the continuous thread was tightened. With the exception of the last group, which applied with no treatments, animals were administered topical formulations of vehicle, extract, or standard drug daily for nine days as described in the grouping and dosage section. The sutures were taken out on day 8 following the incision, and the therapy continued until day 9. The degree of healing was subsequently evaluated by measuring the tensile strength on the tenth day.27
To measure the degree of wound healing, the tensile strength—the amount of force needed to split healing skin—was employed. On the tenth day following injury, each mouse was given high dose of ketamine as anesthesia in order to restrain it to the table. The two forceps were forcefully used on the skin adjacent to the incision, 1 cm apart from the area that had healed. One of the forceps was firmly fixed, and the other was attached to a lightweight, free-floating plastic bag container by a line that had been run over to a pulley. The IV line is opened, allowing water from the faucet to flow into the bag. The incision site gradually came under the weight, pulling the borders of the wound apart. Water flow was stopped as soon as wound gaping developed, and the amount of water gathered in the container was calculated and reported as an indirect measurement of breaking strength in grams.27 The formula below was used to measure tensile strength.
% Tensile of strength (TS) extract = TS extract - TS s.o X 100
TS s.o
% Tensile of strength (TS) of reference = TS reference -TS s.o X 100
TS s.o
%Tensile strength (TS) of s.o= TS s.o –TS l.u X 100
TS l.u
where TS is tensile strength, s.o is simple ointment and lu is left untreated
Anti-Inflammatory Activity
A model of paw edema caused by carrageenan was used to test the extract’s acute anti-inflammatory efficacy.28 After a night of fasting with free access to water, the basal volume of each rat’s left hind paw was measured using a plethysmometer (Ugo Basile, Italy) before any medication was given. The basal volume was established, and the animals were divided into five groups with comparable mean volumes. Standard (Aspirin, 150 mg/kg), vehicle (distilled water), and extract (100 mg/kg, 200 mg/kg, and 400 mg/kg) were all given orally by gavage. After an hour, the animals received an injection in the left hind paw’s sub plantar region with 0.05 mL of a solution of 1% carrageenan in 0.9% saline (w/v) to cause inflammation. Following the injection of carrageenan, the paw volume was measured at one, two, three, and four hours. Based on the difference in paw edema following and prior to (base volume) carrageenan administration, the volume of edema was determined for each rat.29 Using the procedure indicated elsewhere,30 the percentage inhibition of edema for each group was computed.
Percentage inhibition of edema = Co-Ct X100
Co
where Ct represents the average inflammation in rats treated with aspirin or a plant extract at the same time as Co represents the average inflammation (hind paw edema) in the treated rats.
Antioxidant Activity
Using the method31 described elsewhere, the 2, 2-diphenyl-2-picrylhydrazyl free radical (DPPH) assay was used to determine the plant extracts free radical scavenging action. At concentrations of 0.5, 0.25, 0.125, and 0.065 mg/mL, test samples were successively diluted in methanol. After creating a standard stock solution of ascorbic acid in methanol at a concentration of 0.5 mg/mL, serial dilutions of 0.25, 0.125, and 0.0625 mg/mL were prepared. Test tubes containing different amounts of test solution, ascorbic acid solution, and methanol were each given five milliliters of 0.004% DPPH solution (4mg/1000mL in methanol), and the reaction was allowed to proceed at 37°C in the dark for 30 minutes. The absorbance was determined at 517nm using a UV spectrophotometer (Jenway Model 6500, England). The results of each experiment were averaged after being performed in triplicate. The concentration vs percent inhibition graph was used to determine the IC50 value, which was then used to demonstrate DPPH scavenging activity. The sample concentration needed to scavenge 50% of the DPPH free radicals is indicated by the IC50 value. The relationship between antioxidant activity and IC50 is linear. To calculate the free radical scavenging activity (percent antiradical activity), the following equation was used:
% of Inhibition = (Absorbance of control -Absorbance of Test) x100
Absorbance of control
Ascorbic acid was employed as a positive control, along with the DPPH solution and methanol as a negative control.
Total Phenolic Content
To calculate the total phenol content, the Folin-Ciocalteu method was employed. Gallic acid at concentrations of 1, 0.5, 0.25, and 0.125 mg/mL was serially diluted in methanol to create a calibration curve. Then test tubes received one milliliter of gallic acid. Next, 5 milliliters of distilled water and 0.5 milliliters of 2N Folin-Ciocalteu’s-reagent were added to the test tubes (1:20). Following the addition of 2 mL of Na2CO3 (7.5%) after 8 minutes, distilled water was added until the solution level reached 10 milliliter. The solution was then left at room temperature for an additional 30 minutes. Using a UV-Vis spectrophotometer, the solution’s absorbance was measured at 765 nm (Jenway Model 6500, England). The experiment was carried out three times, with the average outcome being used. The extract (0.5mg/mL) and the control solution were prepared in a similar manner. Gallic acid equivalent milligrams per gram of extract served as a measure of the total phenolic content.32
Total Flavonoid Content
The total flavonoid content of the extracts was assessed using an assay for the formation of aluminum chloride complexes. By making sequential dilutions of quercetin in methanol at concentrations of 0.5, 0.25, 0.125, and 0.065 mg/mL, a calibration curve was produced. One milliliter of quercetin was then diluted and put into test tubes. 5% NaNO2 (0.3 milliliter) was added and allowed to sit for 5 minutes. The solution received an additional 0.3 milliliter of 10% AlCl3 and was allowed to stand for 5 minutes. The solution was then given 2 milliliter addition of a 1M NaOH solution, followed by addition of distilled water up to 10 milliliter. The combination was then allowed to sit at room temperature for 30 minutes. Using a UV-Vis spectrophotometer, the solution’s absorbance was measured at 510 nm (Jenway Model 6500, England). Both the blank solution and the extract (0.5mg/mL) underwent the same procedures. The extract’s total flavonoid content was stated as mg of quercetin equivalent per 1g of extract. After three iterations of the experiment, the average output was used.33
Statistical Analysis
The experiment’s results were expressed as mean ± SEM (standard error of the mean). Using SPSS version 25 software, the results were statistically analyzed using one-way analysis of variance (ANOVA) and the Post Hoc Tukey’s test. Data were considered statistically significant at p < 0.05.
Results
Acute Dermal Toxicity Test
This study set out to determine whether a single topical application of the extract could irritate the skin. None of the animals showed any evidence of inflammation, edema, or other behavioral changes over the course of the experiment.
Excision Wounds
Wound Contraction
When mice were applied with an ointment containing an 80% methanol extract of C. simensis leaves, the process of wound healing was significantly enhanced (p < 0.001) compared to the initial wounding (Figure 1). Table 1 displays the development of wound contraction brought on by application of nitrofurazone 0.2% (w/w) ointment, simple ointment base, and 5% (w/w) and 10% (w/w) ointments containing 80% methanol extract.
Figure 1.
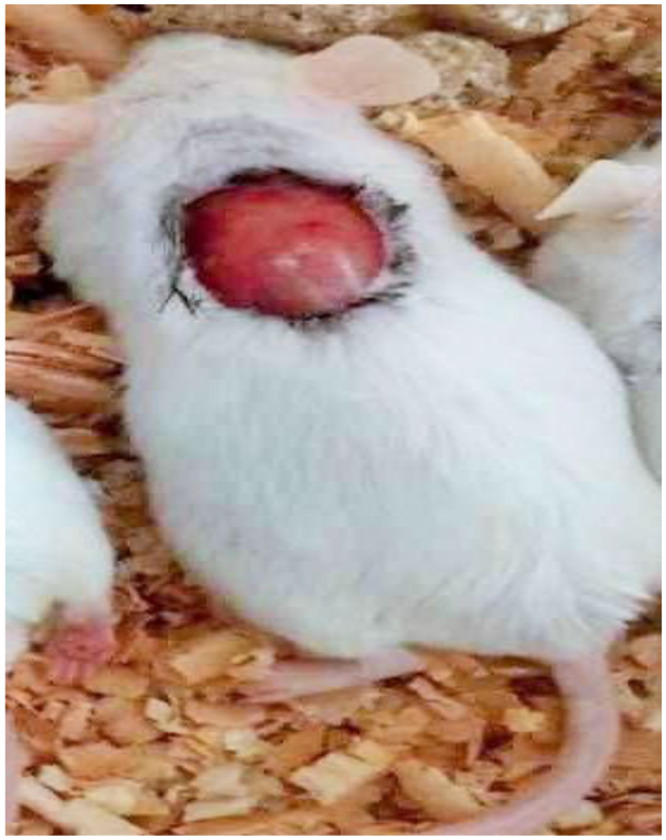
Photograph of excision wound model on day 0.
Table 1.
Effect of Topical Application of 80% Methanol Leaf Extract of C. Simensis on Excision Model in Mice
| Groups | Wound Area (Mm2) Post Wounding Day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | |
| Simple ointment | 290.9±2.9 (3.03%) | 287.1±3.3 (4.28%) | 265.3±9.9 (11.55%) | 166.4±17.6 (44.53%) | 146.6±23.1 (51.13%) | 95.2±9.1 (68.25%) | 56.6±3.6 (81.14%) | 23.7±2.4 (92.09%) | 7.4±1.8 (97.54%) |
| CS 5% | 287.7±5.3 (4.11%) | 264.1±4.8 (11.96%) | 222.8±7.5 (25.71%)e1 | 86.6±9.0 (71.14%)e2 | 37.1±4.0 (87.61%)e3 | 19.2±3.0 (93.6%)e3 | 9.6±1.2 (96.78%)e3 | 2.1±0.1 (99.28%)e3 | 0 (100%) |
| CS 10% | 287.7±5.3 (4.11%) | 267.5±12.1 (10.83%) | 241.7±7.3 (19.42%) | 109.9±19.9 (63.37%) | 41.9±5.4 (86.02%)d3 | 22.9±3.5 (92.34%)d3 | 11.2±3.3 (96.28%)d3 | 5.3±2.1 (98.23%)d3 | 0 (100%) |
| Nitrofurazone 0.2% | 249.8±16.1 (16.73%)a1b1c1 | 235.9±15.2 (21.35%)a1 | 204.1±10.9 (31.98%)a2c1 | 69.0±20.9 (76.98%) | 31.5±3.3 (89.49%)a3 | 12.7±1.4 (95.76%)a3 | 2.7±1.3 (99.11%)a3 | 0 (100%) | 0 |
Notes: Values are expressed as mean ± SEM (n=5 animals in each group) and analyzed by one way ANOVA followed by Tuckey post hoc test; a-“nitrofurazone vs simple ointment”, b- “nitrofurazone vs CS5%”, c- “nitrofurazone vs CS10%”, d- “CS10% vs simple ointment”, e- “CS5% vs simple ointment”; CS, Clematis simensis ointment at concentration of 5% and 10%; 1 - “p < 0.05”, 2- “p < 0.01”, 3- “p < 0.001”.
Starting on the sixth day, the group that applied with the 5% (w/w) crude extract ointment demonstrated a significant (P < 0.05) wound contraction. From the 10th day onwards, the wound healing was significantly different from the negative control group (P < 0.001) than the simple ointment. From the 10th day forward, the group that applied with 10% (w/w) crude extract ointment displayed wound contraction, which was highly significant (P < 0.001) compared to the negative control group (simple ointment). According to Table 1, there was no discernible difference between the 10% (w/w) and 5% (w/w) extracts in terms of wound healing activity.
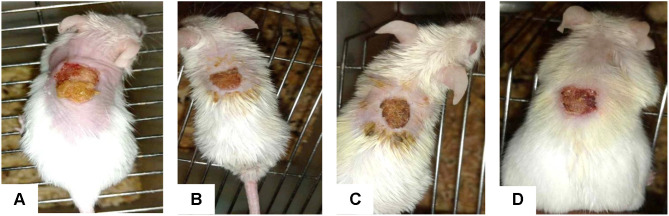
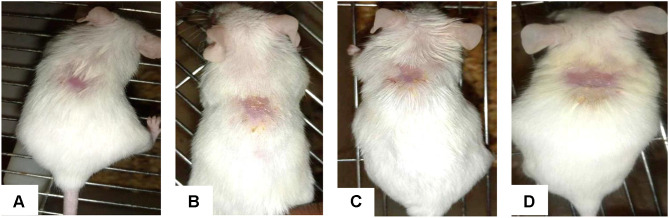
When compared to the control group, the animals treated with 5% (w/w) and 10% (w/w) crude extract ointment significantly reduced wound size by the eighth day (p < 0.05) (Figure 2). From the second day, the group treated with nitrofurazone 0.2% (w/w) ointment showed a significant (p < 0.05) wound contraction. From the tenth day forward, this effect was significantly different from the control group (p < 0.001) than before (Figure 3).
Figure 2.
Photograph of excision wound test result for negative control (A), 5% (B) and 10% extract (C) and nitrofurazone (D), respectively, on day 8.
Figure 3.
Photograph of excision wound test result for negative control (A), 5% (B) and 10% extract (C) and nitrofurazone (D), respectively, on the last day (the day of wound closure for each group).
The highest rates of wound contraction—86.02%, 92.34%, and 96.28%, respectively—were seen in animals applied with 10% extract ointment on the 10th, 12th, and 14th days. Animals treated with conventional medication on days 10, 12, and 14 showed comparable percentages of wound contraction (89.49%, 95.76%, and 99.11%). There was a modest difference between the two groups (5% and 10%) and the standard medicine, despite the fact that there was no discernible variation in the wound healing activity between the two groups (5% and 10% extracts). On day 18, groups treated with ointments containing 5% (w/w) extract and 10% (w/w) extract showed full wound closure. However, for the standard drug complete wound closure was observed on day 16.
Epithelialization Period
In comparison to the untreated groups, those who were treated with extract ointment and nitrofurazone saw complete epithelialization relatively soon (simple ointment treated group). The duration of epithelialization was, respectively, 20.40, 16.80, 16.00, and 13.20 for the negative control group, 5% (w/w) extract ointment, and 10% (w/w) standard medication.
When compared to the control group, the regular medication and the 10% (w/w) ointment both demonstrated a significantly shorter epithelialization duration (p < 0.01). Additionally, when compared to the other groups, the 0.2% nitrofurazone group displayed a highly significant (p < 0.001) duration of epithelialization. The 5% (w/w) extract treatment group lacked statistically significant effect compared to the control group. There was no discernible difference in the duration of epithelialization between the positive control and the extract at 5% (w/w) and 10% (w/w) (Table 2).
Table 2.
Effect of Topical Application of 80% Methanol Leaf Extract Ointment of C. Simensis on Period of Epithelialization
| Group | Period of Epithelialization (Days) Mean ± SEM |
|---|---|
| Simple ointment | 20.40 ± 0.97 |
| CS 5% | 16.80 ± 1.20 |
| CS10% | 16.00 ± 1.09* |
| 0.2% Nitrofurazone | 13.20 ± 0.48*** |
Notes: Values are expressed as mean ± SEM (n = 5 animals in each group) and analyzed by one way ANOVA followed by Tuckey post hoc test; CS, Clematis simensis ointment at concentration of 5% and 10%; *- “p < 0.05”, ***- “p < 0.001”.
Incision Wounds
Despite the fact that all of the incised and sewn wound (Figure 4) were healed, the recorded tensile strength were variable. As indicated in Table 3, when compared to the untreated group, the standard medication, 10% (w/w) extract, and 5% (w/w) extract topically treated groups each shown a significant increase in breaking strength p < 0.05.
Figure 4.
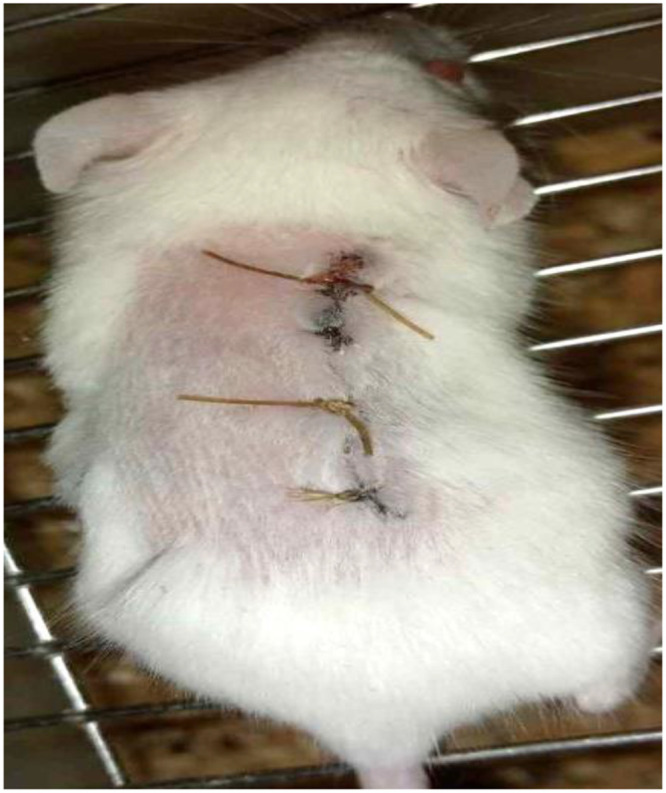
Photograph of Incision wound on day 0.
Table 3.
Effect of Topical Application of the 80% Methanol Leaf Extract Ointment of C. Simensis on Tensile Strength in Incision Wound Model
| Group | Tensile Strength in Gram (Mean ± SEM) | % Tensile Strength |
|---|---|---|
| Untreated group | 235.46 ± 51.49 | |
| Simple ointment | 289.67 ± 43.52 | 23.02% |
| CS 5% | 416.48 ± 17.68 d1 | 43.77% |
| CS 10% | 448.08 ± 30.66 b1e2 | 54.68% |
| 0.2% Nitrofurazone | 461.96 ± 22.03 a1c2 | 59.47% |
Notes: Values are expressed as mean ± SEM (n = 5 animals in each group) and analyzed by one way ANOVA followed by Tuckey post hoc test; a-“nitrofurazone vs simple ointment”, b- “CS10% vs simple ointment”, c-“nitrofurazone vs untreated group”, d- “CS5% vs untreated group”, e- “CS10% vs untreated group”, CS, Clematis simensis ointment at concentration of 5% and 10%; 1 - “p < 0.05”, 2- “p < 0.01”.
In this finding, nitrofurazone topical treatment led to a greater increase in tensile strength than treatments with 10% and 5% extract ointment. The tensile strength between the groups treated with 10% and 5% extracts was higher, but there was no statistically significant difference between them.
In-vivo Anti-Inflammatory Activity
The doses of 200 mg/kg, 400 mg/kg of the extract, and the group receiving the standard medication all showed notable decreases in edema on the second hour after carrageenan administration when compared to the negative control (Table 4). The standard medication was found to considerably reduce edema (p < 0.01) when compared to the medium dose of the extract (200 mg/kg) and negative control in the final two hours (p < 0.01) and (p < 0.05), respectively, but not at a meaningful level at the lower dose (100 mg/kg). The higher dose (400 mg/kg) of the extract significantly decreased edema in the third (p < 0.01) and fourth (p < 0.05) hours when compared to the negative control.
Table 4.
Anti-Inflammatory Activity of the 80% Methanol Leaf Extract of C. Simensis Using Carrageenan Induced Paw Edema
| Group | Mean Increase in Paw Edema Volume in mL | ||||
|---|---|---|---|---|---|
| Basal | 1h | 2h | 3h | 4h | |
| Negative control | 1.42±0.01 | 1.68±0.04 | 1.89±0.10 | 2.40±0.11 | 2.04±0.06 |
| ASA150 | 1.27±0.02 | 1.49±0.08 (11.3%) | 1.24±0.05 a3b2 (34.39%) | 1.52±0.003 a3c1 (36.67%) | 1.39±0.06 a2c1 (31.86%) |
| CS100 | 1.25±0.09 | 1.59±0.03 (5.36%) | 1.68±0.14 (11.11%) | 1.57±0.02 d3 (34.58%) | 1.69±0.12 (17.15%) |
| CS200 | 1.41±0.11 | 1.61±0.09 (4.17%) | 1.31±0.04 e3g1 (30.68%) | 1.94±0.08 e1g1 (19.16%) | 1.89±0.05 (7.35%) |
| CS400 | 1.16±0.09 f1i1 | 1.41±0.11 f1 (16.07%) | 1.16±0.04 f3h2 (38.62%) | 1.74±0.09 f2 (27.5%) | 1.58±0.12 f1 (22.54%) |
Notes: Values are expressed as mean ±SEM (n=5) and analyzed by one way ANOVA followed by Tuckey post hoc test; a- ASA150 vs negative control, b- ASA150 vs CS100, c- ASA150 vs CS200, d-CS100 vs negative control, e- CS200 vs negative control, f- CS400 vs negative control, g- CS200 vs CS 100, h- CS400 vs CS100, i- CS400vs CS200; 1- p < 0.05; ASA15, Aspirin 150mg/kg; CS, Clematis simensis at doses of 100, 200 and 400 mg/kg; 2- p < 0.01, 3- p < 0.001.
The lower dose (100 mg/kg) at the third hour inhibits the development of edema as compared to the negative control (p < 0.001). A decrease in edema was seen with the medium dose (p < 0.001 at the second hour and p < 0.05 at the third hour).
Significant differences between the low dose (100mg/kg) and the standard medication were present at hour 2 (p < 0.01). The medium dose (200 mg/kg) and the standard medication revealed a significant difference at the third and fourth hour (p < 0.05), while the higher dose (400 mg/kg) did not show a statistically significant difference at all measurement periods.
Antioxidant Activity
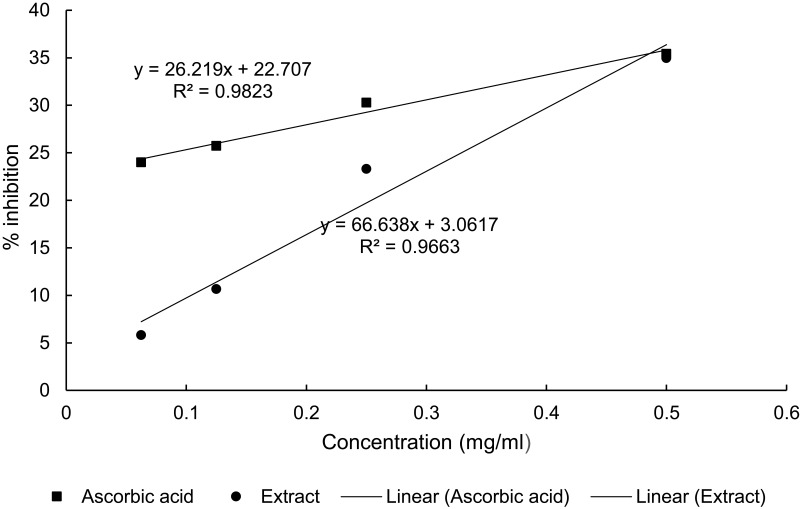
The IC50 values for the extract and ascorbic acid were determined to be 0.70 mg/mL and 1.04 mg/mL, respectively (Figure 5).
Figure 5.
Antioxidant activity of 80% methanol leaf extract of Clematis Simensis. (Values are average of triplicate measurements (mean ± SD).
Preliminary Phytochemical Screening
As indicated in Table 5, a qualitative phytochemical screening of an 80% methanol leaf extract of C. simensis revealed the presence of saponins, tannins, terpenoids, flavonoids, phenols, and steroids; however, alkaloids and steroids were not detected in the extract.
Table 5.
Phytochemical Screening of 80% Methanol Leaf Extract of C. Simensis
| Secondary Metabolite | Results |
|---|---|
| Alkaloids | – |
| Tannins | + |
| Saponins | + |
| Terpeniods | + |
| Flavonoids | + |
| Phenols | + |
| Steroids | – |
Notes: +, present, -, absent.
Total Phenol and Flavonoid Content
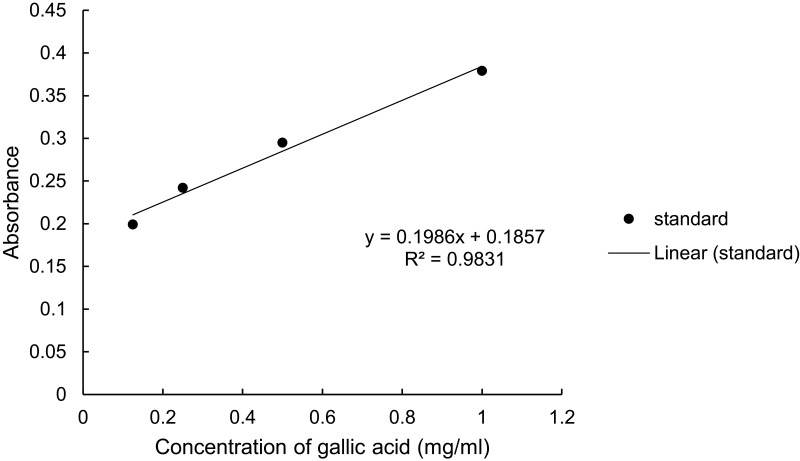
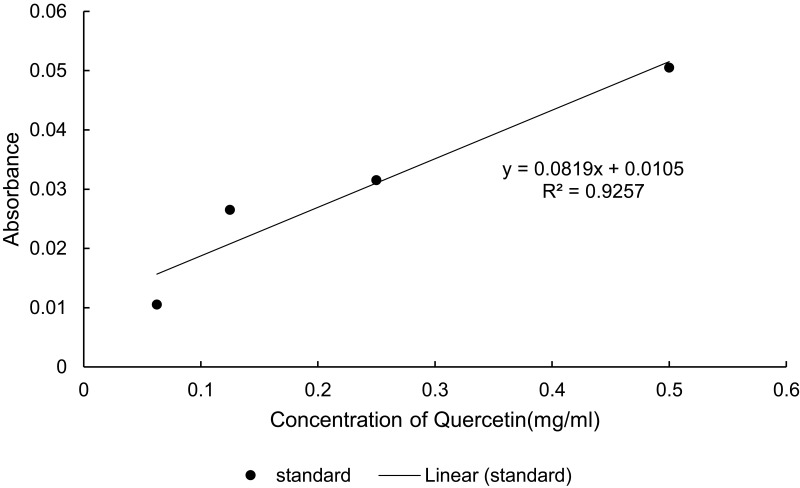
The percentage yield was calculated using the dry extract’s weight and came out to be 23.52% (w/w). The total phenol and flavonoid content of C. simensis leaf extract found from 80% methanol crude extract was determined to be 0.274mg GAE/g (Figure 6) and 0.048mg QE/g (Figure 7), respectively.
Figure 6.
Total phenol content of 80% crude methanol extract of leaf of Clematis simensis.
Figure 7.
Total flavonoid content of 80% crude methanol extract of leaf of Clematis simensis.
Discussion
Because of the intricacy of the wound healing cascade and the difficulty of analyzing the effects of a drug in any in vitro model, this study used both excision and incision in vivo approaches.34 Even though much effort has been put into studying wound healing activity in vitro, in vivo studies are still required to investigate wound healing activity because wound healing is a complex and dynamic process of restoring cellular structure and tissue layers in damaged tissue to their normal state as closely as possible.35
The aim of this study was to evaluate the wound healing, anti-inflammatory and antioxidant activities of the 80% methanol extract of the leaves of C. simensis. In this study, topical treatment with 5% and 10% extract ointment and the standard drug significantly enhanced the rate of wound contraction and epithelialization (Figure 3). It revealed significant wound area contraction starting from the 8th day of wounding and the days forward (Figure 2).
Epithelialization which is an essential component of wound healing is used as a defining parameter of successful wound closure ability.35 As epithelialization progresses, myofibroblast contractile properties improve, epithelial cells proliferate and crawl across the wound bed to cover it, where these proliferation and migration processes, as well as myofibroblast contractile properties, are attributed to the extracts’ significant effect on the epithelialization period (Figure 4).36 The epithelialization time was significantly reduced from 20 days (control) to 16 days for both the 5% and 10% extract (Table 2). The occurrence of enhanced epithelialization and wound contraction could be due to the ability of the extracts to enhance collagen synthesis. Furthermore, the effect might be attributed to the potential of the extracts or its constituents to promote epithelialization either by facilitating proliferation or by increasing the viability of epithelial cells.37
The results of the incision wound model, which showed an increased wound tensile strength on the 10th post wounding day, support the crude extract’s wound healing ability. The breaking strength was calculated as a parameter in the incision wound model to illustrate how well the restored tissue resisted breaking under tension.26 Animal wounds treated with 5% (w/w) extract, 10% (w/w) extract, and 0.2% nitrofurazone ointments increased in breaking strength by 43.77%, 54.6%, and 59.47%, respectively. The percentage of the breaking strength of the simple ointment-treated group, on the other hand, was 23.0%, which was twofold smaller than the percentage of the 10% (w/w) extract ointment. The effect could be due to the presence of secondary metabolites in the test substance such as flavonoids, tannins, saponins, which are responsible for enhancement of collagen maturation which gives strength and integrity to the wound matrix.38 Furthermore, the increment in tensile strength may be associated with the promotion of collagen synthesis, angiogenesis, and stabilization of fibers and hence the overall effect improves circulation of oxygen and nutrient supply that are vital for wound healing cascade.39
The carrageenan-induced hind paw edema model has been widely used in the development and testing of anti-inflammatory medicines.27 The capacity of such medicines to inhibit the edema developed in the hind paw of mice after injection of a phlogistic agent is the basis for this model. The capacity of anti-inflammatory drugs to reduce edema generated in mice paws by carrageenan is one of the most extensively used in vivo animal experiments.29 The present in vivo study revealed anti-inflammatory activity of the 80% methanol leaf extract of C. simensis in carrageenan-induced paw edema model in mice.
This study showed that unlike the higher dose, the lower dose had no significant anti-inflammatory activity during the first and second hour. However, the middle dose and the higher dose showed significant inhibition of edema at the 1st and 2nd hour, which declined at the 3rd and 4th hour when compared with the standard drug. The reason could be the extract might be able to achieve maximum plasma concentration at that specific dose (200mg/kg and 400mg/kg) at the 2nd hour.
Nowadays, the majority of antioxidants are manufactured synthetically. When consumed in vivo, such synthetic antioxidants are known to have potential adverse effects and to be carcinogenic.40 As a result, their use is restricted. However, antioxidant compounds derived from plant materials are harmless and protect organisms from free radical damage, hence encouraging wound healing.41
The antioxidant activity of the extract was evaluated using DPPH free radical scavenging activity. The stable DPPH radical method is a widely used, relatively quick, most accepted and precise method for the evaluation of free radical scavenging activity of a plant extract.31 DPPH is a stable free radical that accepts an electron or hydrogen radical to become a stable diamagnetic molecule. The reduction capability of DPPH radical is determined by the decrease in its absorbance at 517 nm induced by antioxidants. The extract showed strong free radical scavenging activity with IC50 value of 0.7mg/mL (Figure 6).
The preliminary phytochemical analysis of C. simensis extract revealed the presence of flavonoids, terpenoids, saponins, tannins and phenolic compounds (Table 5). These metabolites are usually responsible for the pharmacological activities of medicinal plants.42 Saponins and flavonoids have been reported to possess wound-healing activity.38 Terpenoids are known to enhance wound healing, owing to their astringent and antibacterial properties, which appear to be responsible for wound contraction and higher epithelialization rate.43 Tannins are seen to be active detoxifying agents and inhibit bacterial growth.44
Although this study was able to demonstrate the C. simensis extract’s potential wound healing, anti-inflammatory, and antioxidant activities, it could not identify which metabolites are responsible for the observed results. As a result, the extract’s wound-healing properties can be linked to the phytoconstituents found in it, which may have an additive or individual effect. As a result, it is likely that future research on C. simensis leaf will focus on fractionating, isolating, and characterizing the active constituents, as well as proposing mechanisms of action for them.
Conclusion
The result from the excision and incision models demonstrated that the 80% methanol leaf extract of C. simensis significantly improved wound repair, wound contraction and epithelization. The extracts anti-inflammatory and antioxidant activity perhaps explains the wound healing activity. Therefore, the results obtained justify the use of the leaves of C. simensis for wound healing which upholds the traditional use of the plant.
Acknowledgments
We would like to thank Addis Ababa University College of Health Sciences School of Pharmacy and Ethiopian Public Health Institute for allowing the study to be conducted with facility of the institution.
Abbreviations
ANOVA, analysis of variance; BP, British pharmacopoeia; DPPH, 1, 1-Diphenyl −2 Picryl Hydrazyl; ECM, extracellular matrix; EPHI, Ethiopian public health institute; MMP, matrix metalloproteinases; OECD, organization for economic cooperation and development; SEM, standard error of the mean; SPSS, statistical package for social sciences; WHO, World Health Organization.
Data Sharing Statement
The datasets used and/or analyzed during the current work are available from the corresponding author up on a reasonable request.
Ethics Approval and Consent
The protocol was approved by institutional review board of the School of Pharmacy with Reference no. ERB/SOP/474/14/2022.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kim JY, Dao H. Physiology, Integument. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 2.Gonzalez AC, Costa TF, Andrade ZD, Medrado AR. Wound healing - a literature review. An Bras Dermatol. 2016;91:614–620. doi: 10.1590/abd1806-4841.20164741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oguntibeju OO. Medicinal plants and their effects on diabetic wound healing. Vet World. 2019;12(5):653. doi: 10.14202/vetworld.2019.653-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. 2020;12(8):735. doi: 10.3390/pharmaceutics12080735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MN. The crucial roles of inflammatory mediators in inflammation: a review. Vet World. 2018;11(5):627. doi: 10.14202/vetworld.2018.627-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niederstätter IM, Schiefer JL, Fuchs PC. Surgical strategies to promote cutaneous healing. J Med Sci. 2021;9(2):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai T, Huang YY, Sharma K. Topical antimicrobials for burn wound infections. Recent Pat Antiinfect Drug Discov. 2010;5(2):124–151. doi: 10.2174/157489110791233522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson K, Hamm RL. Factors that impair wound healing. J Am Coll Clin Wound Spec. 2012;4(4):84–91. doi: 10.1016/j.jccw.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw JE, Mitchell C. Dermal drug delivery systems: a review. J Toxicol Cutan Ocul Toxicol. 1983;2(4–5):249–266. doi: 10.3109/15569528309036265 [DOI] [Google Scholar]
- 10.Hosseinkhani A, Falahatzadeh M, Raoofi E, Zarshenas MM. An evidence-based review on wound healing herbal remedies from reports of traditional Persian medicine. J Evid Based Complementary Altern Med. 2017;22(2):334–343. doi: 10.1177/2156587216654773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fikru A, Makonnen E, Eguale T, Debella A, Mekonnen GA. Evaluation of in vivo wound healing activity of methanol extract of Achyranthes aspera L. J Ethnopharmacol. 2012;143(2):469–474. doi: 10.1016/j.jep.2012.06.049 [DOI] [PubMed] [Google Scholar]
- 12.Yesuf A, Asres K. Wound healing and antiinflammatory properties of Allophylus abyssinicus (Hochst.). Radlk Phytopharm. 2013;4(2):442–453. [Google Scholar]
- 13.Mekonnen A, Sidamo T, Asres K, Engidawork E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. J Ethnopharmacol. 2013;145(2):638–646. doi: 10.1016/j.jep.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 14.Mulisa E, Asres K, Engidawork E. Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J. (Polygonaceae) in mice. BMC Complement Altern Med. 2015;15(1):1. doi: 10.1186/s12906-015-0878-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambebo MK, Kifle ZD, Gurji TB, Yesuf JS. Evaluation of wound healing activity of methanolic crude extract and solvent fractions of the leaves of Vernonia auriculifera Hiern (Asteraceae) in mice. J Exp Pharmacol. 2021;13:677. doi: 10.2147/JEP.S308303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tekleyes B, Huluka SA, Wondu K, Wondmkun YT. Wound healing activity of 80% methanol leaf extract of Zehneria scabra (Lf) Sond (Cucurbitaceae) in mice. J Exp Pharmacol. 2021;13:537. doi: 10.2147/JEP.S303808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wondimu T, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants around ‘Dheeraa’town, Arsi Zone, Ethiopia. J Ethnopharmacol. 2007;112(1):152–161. doi: 10.1016/j.jep.2007.02.014 [DOI] [PubMed] [Google Scholar]
- 18.Care IoLARCo, Animals UoL. Guide for the Care and Use of Laboratory Animals: US Department of Health and Human Services. National Public Health Service; 1986. [Google Scholar]
- 19.Morton J, Malone M. Evaluation of vulneray activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther. 1972;196(1):117. [PubMed] [Google Scholar]
- 20.Harborne AJ. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. springer science & business media; 1998. [Google Scholar]
- 21.OECD 404 Guidelines for the Testing of Chemicals. Acute Dermal Irritation/Corrosion. OECD Guidel Test Chem Sect Heal Eff. 1–8. 2015. [Google Scholar]
- 22.Tadele A, Asres K, Melaku D, Mekonnen W. In vivo anti-inflammatory and antinociceptive activities of the leaf extracts of Clematis simensis Fresen. Ethiop Pharm J. 2009;27:33–41. [Google Scholar]
- 23.Kokane DD, More RY, Kale MB, Nehete MN, Mehendale PC, Gadgoli CH. Evaluation of wound healing activity of root of Mimosa pudica. J Ethnopharmacol. 2009;124(2):311–315. doi: 10.1016/j.jep.2009.04.038 [DOI] [PubMed] [Google Scholar]
- 24.Lodhi S, Pawar RS, Jain AP, Singhai AK. Wound healing potential of Tephrosia purpurea (Linn.) Pers. in rats. J Ethnopharmacol. 2006;108(2):204–210. doi: 10.1016/j.jep.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 25.Sharma GN, Dubey SK, Sati N, Sanadya J. Evaluation of wound healing activity of aegle marmelos seed. Pharmacologyonline. 2011;2:171–178. [Google Scholar]
- 26.Shenoy RR, Sudheendra AT, Nayak PG, Paul P, Kutty NG, Rao CM. Normal and delayed wound healing is improved by sesamol, an active constituent of Sesamum indicum (L.) in albino rats. J Ethnopharmacol. 2011;133(2):608–612. doi: 10.1016/j.jep.2010.10.045 [DOI] [PubMed] [Google Scholar]
- 27.Jha RK, Bhandari A, Nema RK. Influence of flower head aqueous extract of Sphaeranthus indicus Linn. on wound healing in albino rats. Am Eur J Sci Res. 2011;6:13–18. [Google Scholar]
- 28.Padilha MM, Vilela FC, Rocha CQ, et al. Antiinflammatory properties of Morus nigra leaves. Phyther Res. 2010;24(10):1496–1500. doi: 10.1002/ptr.3134 [DOI] [PubMed] [Google Scholar]
- 29.Rahman MA, Chakma JS, Islam S, Rana MS, Ahmed NU. Analgesic and anti-inflammatory effect of Clausena suffruticosa root extract in animal model. J Sci Res. 2011;3(3):631–639. doi: 10.3329/jsr.v3i3.7594 [DOI] [Google Scholar]
- 30.Owoyele BV, Nafiu AB, Oyewole IA, Oyewole LA, Soladoye AO. Studies on the analgesic, anti-inflammatory and antipyretic effects of Parquetina nigrescens leaf extract. J Ethnopharmacol. 2009;122(1):86–90. doi: 10.1016/j.jep.2008.11.027 [DOI] [PubMed] [Google Scholar]
- 31.Mahdi-Pour B, Jothy SL, Latha LY, Chen Y, Sasidharan S. Antioxidant activity of methanol extracts of different parts of Lantana camara. Asian Pac J Trop Biomed. 2012;2(12):960–965. doi: 10.1016/S2221-1691(13)60007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.María R, Shirley M, Xavier C, et al. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J King Saud Univ Sci. 2018;30(4):500–505. doi: 10.1016/j.jksus.2017.03.009 [DOI] [Google Scholar]
- 33.Nigatu H, Belay A, Ayalew H, et al. In vitro antileishmanial activity of some Ethiopian medicinal plants. J Exp Pharmacol. 2021;13:15–22. doi: 10.2147/JEP.S285079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masson‐Meyers DS, Andrade TA, Caetano GF, et al. Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol. 2020;101(1–2):21–37. doi: 10.1111/iep.12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. doi: 10.1126/scitranslmed.3009337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samanta R, Pattnaik AK, Pradhan KK, Mehta BK, Pattanayak SP, Banerjee S. Wound healing activity of silibinin in mice. Pharmacognosy Res. 2016;8(4):298. doi: 10.4103/0974-8490.188880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee H, Ojha D, Bharitkar YP, et al. Evaluation of the wound healing activity of Shorea robusta, an Indian ethnomedicine, and its isolated constituent (s) in topical formulation. J Ethnopharmacol. 2013;149(1):335–343. doi: 10.1016/j.jep.2013.06.045 [DOI] [PubMed] [Google Scholar]
- 38.Garg VK, Paliwal SK. Wound-healing activity of ethanolic and aqueous extracts of Ficus benghalensis. J Adv Pharm Technol Res. 2011;2(2):110. doi: 10.4103/2231-4040.82957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arun M, Satish S, Anima P. Evaluation of wound healing, antioxidant and antimicrobial efficacy of Jasminum auriculatum Vahl. Avicenna J Phytomed. 2016;6(3):295–304. [PMC free article] [PubMed] [Google Scholar]
- 40.Lourenço SC, Moldão-Martins M, Alves VD. Antioxidants of natural plant origins: from sources to food industry applications. Molecules. 2019;24(22):4132. doi: 10.3390/molecules24224132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy G. In vitro evaluation of antioxidant activity of methanolic extracts of selected mangrove plants. Med Aromat Plants. 2016;5(3):548. [Google Scholar]
- 42.Nair R, Kalariya T, Chanda S. Antibacterial activity of some selected Indian medicinal flora. Turk J Biol. 2005;29(1):41–47. [Google Scholar]
- 43.Umeh VN, Ilodigwe EE, Ajaghaku DL, Erhirhie EO, Moke GE, Akah PA. Wound-healing activity of the aqueous leaf extract and fractions of Ficus exasperata (Moraceae) and its safety evaluation on albino rats. J Tradit Complement Med. 2014;4(4):246–252. doi: 10.4103/2225-4110.139105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung KT, Wong TY, Wei CI, Huang YW, Lin Y. Tannins and human health: a review. Crit Rev Food Sci Nutr. 1998;38(6):421–464. doi: 10.1080/10408699891274273 [DOI] [PubMed] [Google Scholar]