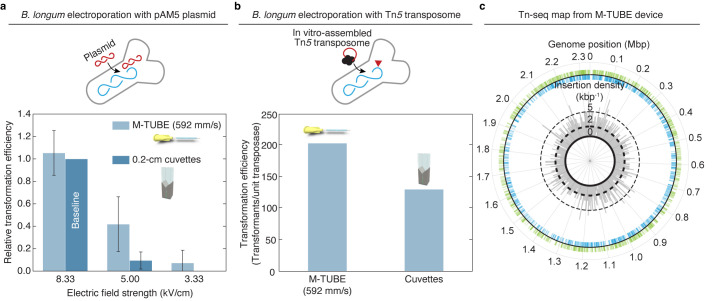
Fig 3. M-TUBE efficiently transforms anaerobic bacteria and enables transposon insertion mutagenesis.
(a) Comparison of M-TUBE performance during electrotransformation of B. longum NCIMB8809 with the plasmid pAM5 at various electric field strengths. For M-TUBE devices, voltages of ±2.50, ±1.50, and ±1.00 kV (AC field) were applied to produce electric fields of 8.33, 5.00, and 3.33 kV/cm, respectively. A fluid velocity of 592 mm/s was used for the M-TUBE device because approximately 5 ms residence time with an M-TUBE ID of 0.5 mm is similar to the time constant observed in cuvette electroporation (5.2–5.6 ms). Data represent the average (n ≥ 3) and error bars represent 1 standard deviation. (b) Comparison of M-TUBE performance during electrotransformation of B. longum NCIMB8809 with Tn5 transposome. For the M-TUBE device, a field strength of 8.33 kV/cm and fluid velocity of 592 mm/s were used, motivated by the results in (a). (c) The transposon insertions recovered from Tn5 transposome electroporation are spread approximately uniformly across the B. longum NCIMB8809 genome. The locations of individual mapped insertions are recorded on the outer circle. Green ticks on the outside indicate insertions in the positive (+) orientation, blue ticks on the inside indicate insertions in the negative (−) orientation. The insertion density (kbp−1) (both positive and negative orientation) is plotted in 1-kbp bins on the inner circle. Transposon insertions are distributed throughout the genome in both the positive and negative orientations, indicating that B. longum NCIMB8809 can be transformed by Tn5 transposomes using M-TUBE without major insertional bias. The data underlying this figure can be found in S2 Data. AC, alternating current; ID, inner diameter; M-TUBE, microfluidic tubing-based bacterial electroporation.