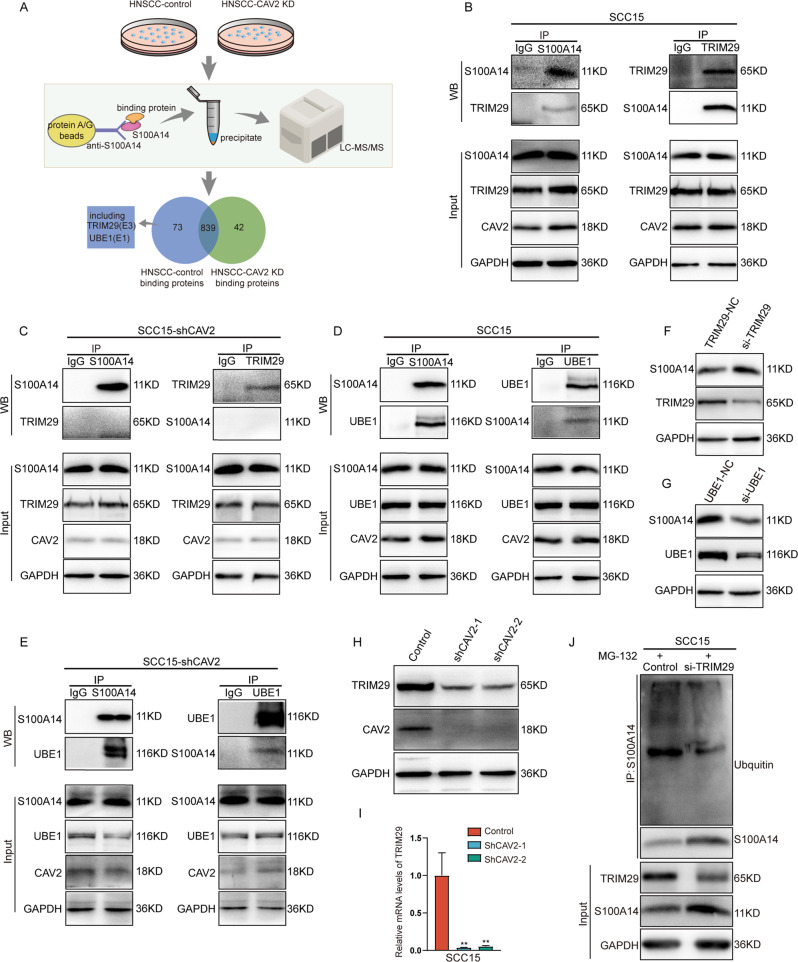
Fig. 6. CAV2 promotes S100A14 ubiquitylation and degradation through TRIM29.
A Immunoprecipitation assays and liquid chromatography–mass spectrometry (LC–MS) analysis of wild-type SCC15 and CAV2-deficient SCC15 cells. B SCC15 cell lysates were subjected to immunoprecipitation (IP) with anti-S100A14 or anti-TRIM29 antibodies, followed by western blotting of the immunoprecipitates with antibodies against S100A14 and TRIM29, as indicated. C shCAV2 SCC15 cell lysates were subjected to immunoprecipitation (IP) with anti-S100A14 or anti-TRIM29 antibodies, followed by western blotting of the immunoprecipitates with antibodies against S100A14 and TRIM29, as indicated. D SCC15 cell lysates were subjected to immunoprecipitation (IP) with anti-S100A14 or anti-UBE1 antibodies, followed by western blotting of the immunoprecipitates with antibodies against S100A14 and UBE1, as indicated. E shCAV2 SCC15 cell lysates were subjected to immunoprecipitation (IP) with anti-S100A14 or UBE1 antibodies, followed by western blotting of the immunoprecipitates with antibodies against S100A14 and UBE1, as indicated. F The protein levels of TRIM29 and S100A14 were examined by immunoblotting in SCC15 cells transfected with TRIM29-siRNA and control-siRNA for 48 h. G The protein levels of UBE1 and S100A14 were examined by immunoblotting in SCC15 cells transfected with UBE1-siRNA and control-siRNA for 48 h. H, I The protein or mRNA expression of TRIM29 in CAV2-control and CAV2-knockdown SCC15 cells was evaluated by immunoblotting or RT–qPCR. J Ubiqutin-S100A14 was detected by immunoprecipitation (IP) using anti-S100A14 with a subsequent immunoblot assay with anti-ubiquitin antibody in control and siTRIM29 SCC15 cells.