Figure 3.
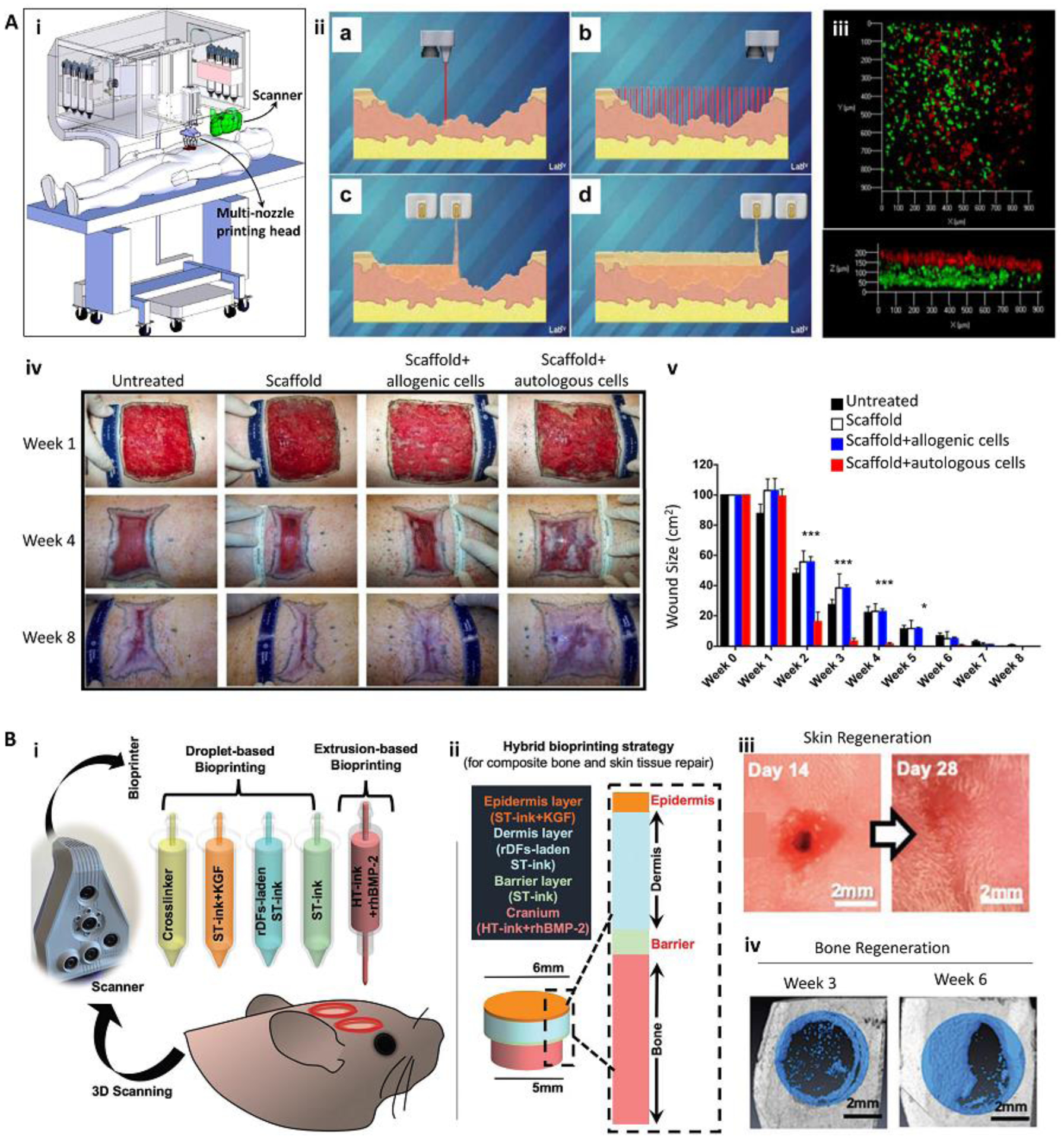
In situ bioprinting for the treatment of large and complex tissue defects. (A) In situ bioprinting for treatment of large burn wounds. The printing approach was based on integrated scanning and multimaterial inkjet printing (i). The scanner was first used to reconstruct the defect morphology, followed by deposition of fibroblast-laden dermal and keratinocyte-laden epidermal layers (ii, iii). Fibroblasts (green) and keratinocytes (red) layers formed in vitro (iii). An in situ bioprinting on porcine burn wounds demonstrated a rapid wound closure and reduced contraction when autologous cells were encapsulated in the bioink (iv, v). (B) The treatment of complex bone/skin composite defect with a hybrid in situ bioprinting approach. Scanning was used to reconstruct the defect geometry, while extrusion and inkjet printing methods were implemented for in situ printing of high viscosity acellular bone and low viscosity cellular skin bioinks, respectively (i, ii). Gross pictures of skin (iii) and bone (iv) tissue regeneration over 6 weeks post-surgery demonstrate major recovery of composite tissue. ST-ink: soft tissue ink consisting from collagen and fibrin; KGF: keratinocyte growth factor; rDF: rat primary dermal fibroblasts; HT-ink: hard tissue ink consisting from collagen, chitosan, nano-hydroxyapatite particles (nHAp), and β-Glycerophosphate disodium salt (β-GP); rhBMP2: recombinant human bone morphogenetic protein-2. Reproduced with permission from Nature Publishing Group [4] (A) and Wiley [38] (B).
