Figure 4.
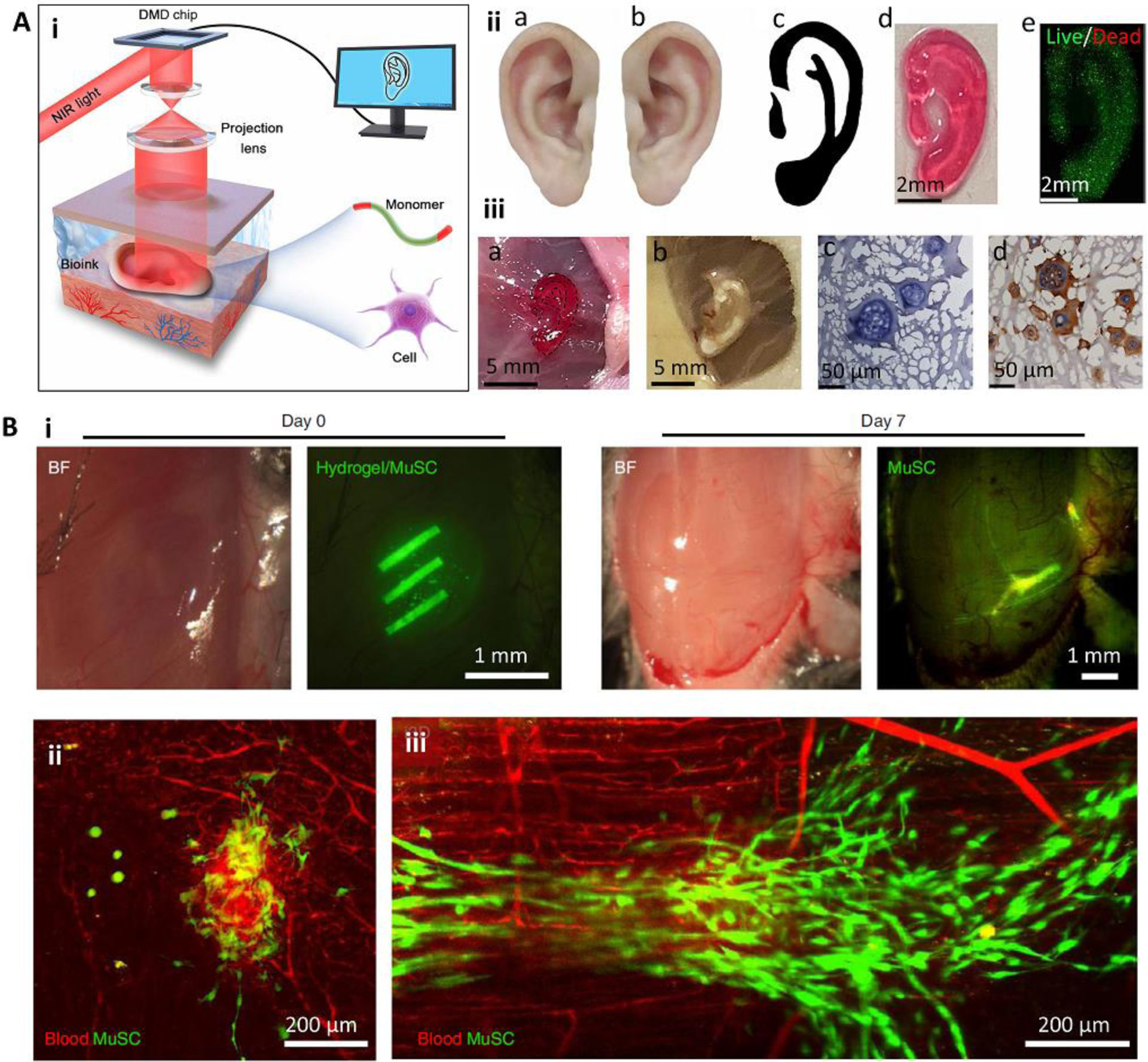
Minimally invasive in situ SLA bioprinting. (A) The application of digital light processing for subcutaneous in situ bioprinting. A DMD chip was used to project NIR light through the intact skin and crosslink pre-injected cell-laden bioink (i). The in vitro (ii) and in vivo (iii) formation of an ear-like structure through intact skin. In this bioprinting strategy, the healthy tissue is scanned and mirrored to provide a representative model of the defected tissue (iia-iic). The model is then sliced and printed layer-by-layer. A fine structure with high cell viability could be achieved (iid, iie). The printed structure (iiia) was stable after 1 month (iiib), and demonstrated tissue integration by H&E staining (iiic) and immunostaining of collagen II (iiid) secreted by chondrocytes encapsulated in the printed structure. (B) Intramuscular bioprinting of scaffolds embedding muscle-derived stem cells. Elongated structures were printed to mimic the structure of native muscle (i). Results obtained after 7 days post-operation demonstrated that despite the injection of the bioink without subsequent selective crosslinking (ii), the injection of the bioink followed by selective crosslinking for the formation of elongated structures can induce organized muscle cell (green) architectures aligned with blood vessels (red). Reproduced with permission from the American Association for the Advancement of Science [37] (A) and Nature Publishing Group [36] (B).
