Figure 5.
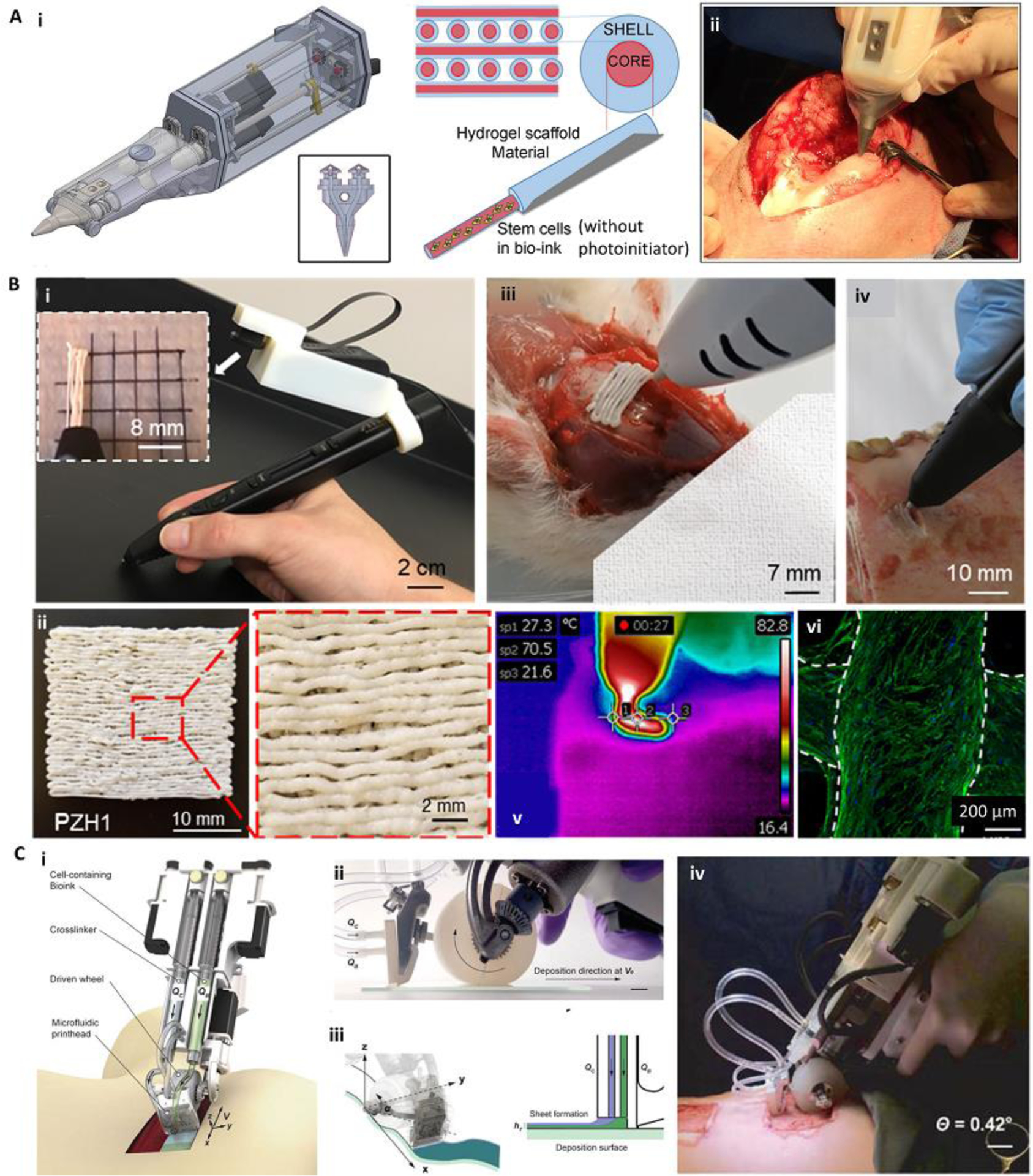
Various handheld in situ printing strategies. (A) An extrusion-based handheld bioprinter for deposition of core-shell filaments for the treatment of osteochondral defects. The device (Bio Pen) could print a cell-laden core and a protective acellular shell (i). The Bio Pen was implemented for printing into a full-thickness osteochondral defect in the knee of a sheep (ii). (B) A melt-spinning handheld printer for treatment of bone defects. The handheld printer could be used to melt PCL-based material and deposit its filaments with a fine resolution, while a camera was integrated for better visibility (i, ii). The printer was used to fill ex vivo murine calvarial and porcine jaw defects (iii, iv). The temperature of the melt-extrusion filaments was shown to be lower than the threshold to induce cell death at the tissue filament interface (v). The printed scaffolds were reported to be osteoinductive after in vitro seeding (vi). Osteopontin (green) and nuclei (blue) were immunostained on human mesenchymal stem cells differentiated into osteoblasts for 28 days. (C) An extrusion-based handheld planar printer for treatment of large burn wounds. The device could co-deposit sheets of bioink and its crosslinker to fill and conform to clinically sized and shaped skin defects (i). The planar printer had a silicone wheel to control the relative velocity of the nozzle over the defect (ii). The printer enabled two degrees of spatial control to lay down the bioink and crosslinker along non-uniform edges of a skin wound (iii). The planar printer was used for the treatment of angled porcine full-thickness burn injuries (iv). Adapted with permission from Wiley [49] (A), Elsevier [46] (B), and IOP publishing [45] (C).
