Fig.6: Models summarizing the multiple locations of RB and its changing role during cell cycle progression.
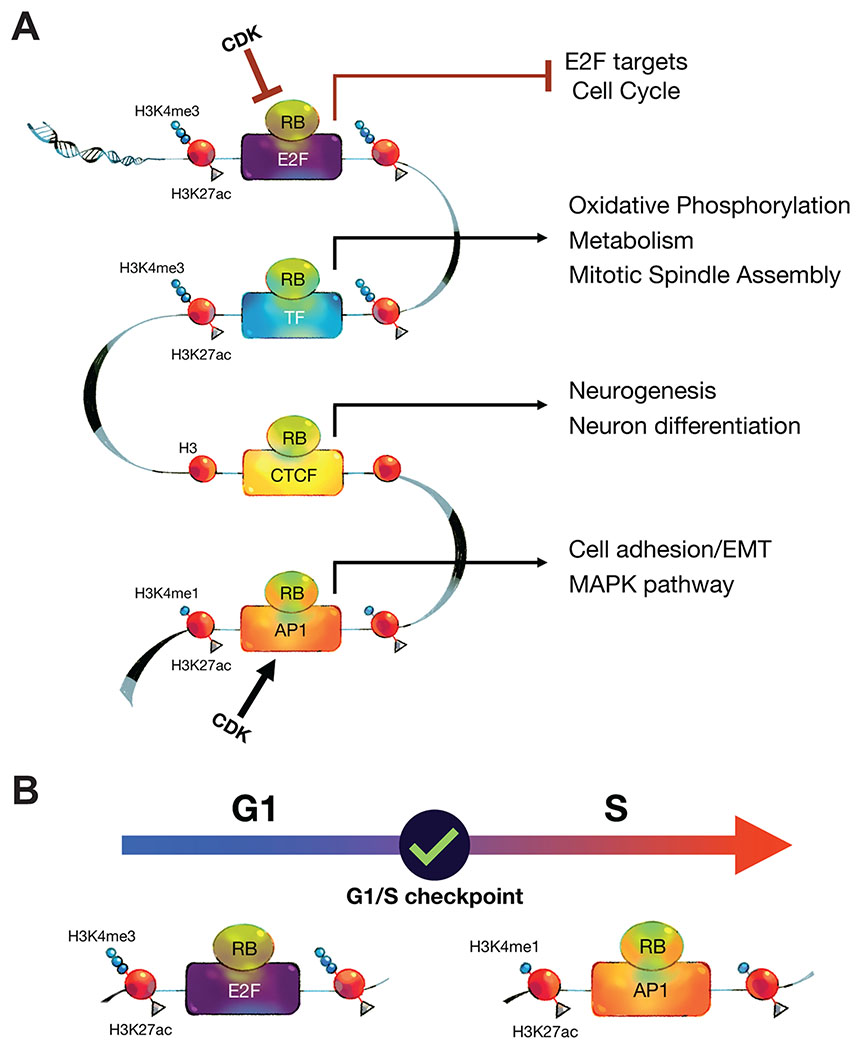
(A) RB associates with three distinct chromatin regions: promoters, insulators, and enhancers. E2F/DP1 mediates RB recruitment to E2F-promoters, an interaction that is stronger when cells accumulate in G1. These RB/E2F promoters regulate E2F target genes involved in cell cycle regulation. A subset of RB-bound promoters that is less enriched in E2F motifs but shows strong enrichment in motifs of other TFs, such as Sp1 and ETS family of TFs. This second group of RB-bound promoters regulates genes involved in metabolic pathways and mitotic spindle assembly. RB/CTCF insulator sites are CTCF-bound chromatin regions that are also bound by RB. These sites are depleted of both promoter and enhancer histone marks. Genes in proximity to RB/CTCF sites are often associated with neuronal differentiation. In enhancer regions, RB is recruited by AP-1 transcription factors, and the recruitment is inhibited when cells accumulate in G1. Genes that are linked to RB-enhancer sites are involved in cell adhesion and MAPK pathway regulation in RPE1 cells. (B) The “check ‘n go” functions of RB. RB has two separate roles during the cell cycle. In G1, RB represses E2F promoters and checks CDK activity before allowing cells to enter S-phase. RB binding to these regions is highly conserved across different cell types, enabling its “check” function in most cell types. However, when the cell makes the decision to divide, cell type-specific programs are needed to ensure the appropriate fate of the daughter cells. After the G1/S checkpoint, the role of RB changes, and it moves to enhancers, where it regulates transcriptional programs that are cell type-specific.
