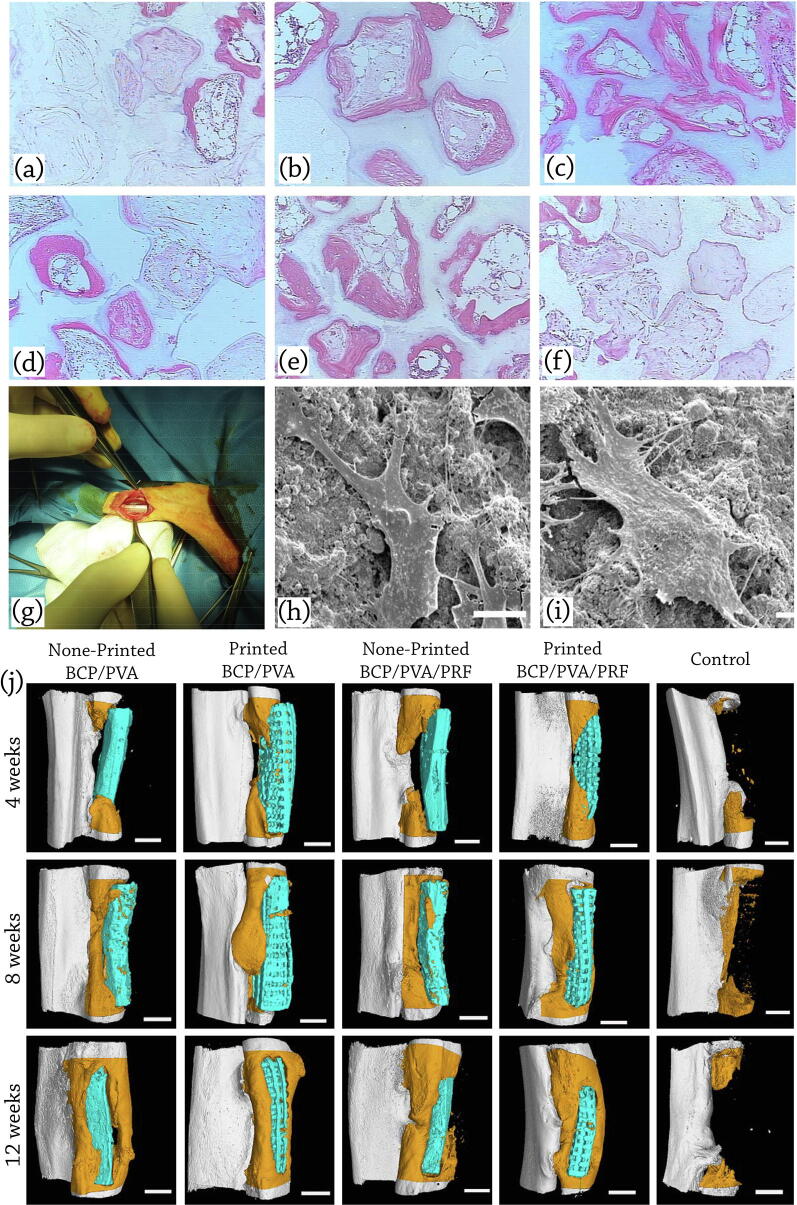
Fig. 7.
In-vivo applications of BCP scaffolds: representative histological micrographs of (a) 100 HA, (b) 76 HA/24 β-TCP, (c) 63 HA/37 β-TCP, (d) 56 HA/44 β-TCP, (e) 20 HA/80 β-TCP, and (f) 100 β-TCP at 12 weeks post implantation (H&E staining). Bone is stained pink, ceramic has a shadowy white appearance, loose connective tissue is stained a light pink, cells are stained a dark pink, reproduced with permission from [190]. Seeding hMSCs on BCP samples with HA: β-TCP composition ratio of 20: 80 showed the fastest rate of bone induction in a mouse ectopic model among all examined samples. (g) Implanted scaffold in the radius defect of the rabbit; reproduced with permission from [172]. (h) SEM analysis of the adhesion status of BMSCs on the printed BCP/PVA scaffolds (i) printed BCP/PVA/PRF scaffolds, reproduced with permission from [178] (j) Micro-CT scans and 3D reconstructions to visualize healing of critical size bone defects in the rabbit radius at 4, 8, and 12 weeks after implantation of scaffolds seeded with BMSCs. Defects were implanted with non-printed BCP/PVA scaffolds, printed BCP/PVA scaffolds, non-printed BCP/PVA/PRF scaffolds, printed BCP/PVA/PRF scaffolds, and the sample with no following treatment (control). The scaffolds are shown in blue, and newly formed radius bone is shown in dark orange in the micro-CT images; scale bars: 3 mm, reproduced with permission from [178]. The incorporation of PRF, promoted the biological activity through enabling the sustained release of bioactive factors from the scaffold.