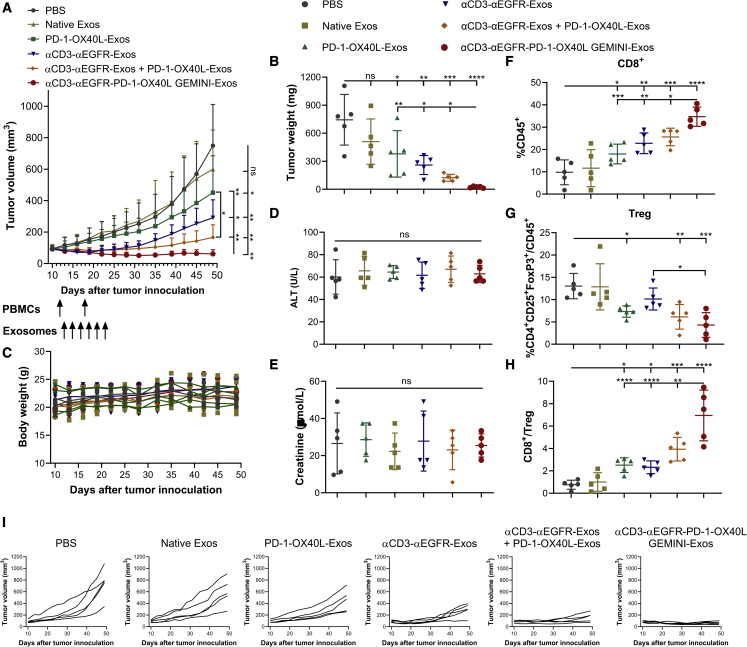
Figure 4.
In vivo evaluation of αCD3-αEGFR-PD-1-OX40L GEMINI-Exos
(A) Anti-tumor activity of GEMINI-Exos. BT-20 cells were subcutaneously implanted into the flank of female NSG mice (n = 5). In vitro expanded human PBMCs from the same healthy donor were intraperitoneally injected into mice on days 12 and 18 post tumor implantation. One day post the first PBMC administration, mice were treated with PBS or different types of exosomes (10 mg/kg for monotherapy and 20 mg/kg for combination therapy) every other day for a total of six times via intravenous injections. Data are shown as mean ± SD (n = 5). ns = not significant, ∗p < 0.05, and ∗∗p < 0.01(one-way repeated measures ANOVA test with the Geisser-Greenhouse correction). (B) Tumor weights at the end of study. (C) Body weights of mice during the study. (D) ALT activities in plasma at the end of study. (E) Creatinine concentrations in plasma at the end of study. (F) Percentage CD8+ T cells in CD45+ cells in tumors. (G) Percentages of CD4+ CD25+ FoxP3+ Tregs in CD45+ cells in tumors. (H) CD8+ T cell/Treg ratios in tumors. At the end of the study, tumors were harvested and disaggregated into single-cell suspensions. After immunostaining, cells were analyzed by flow cytometry for the expression of CD45, CD4, CD8, CD25, and FoxP3. (I) Tumor growth curves for individual mice during the study. Data in (B, D–H) are shown as mean ± SD (n = 5). ns = not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001 (ordinary one-way ANOVA test).