Abstract
It is well established that macrophages are key regulators of wound healing, displaying impressive plasticity and an evolving phenotype, from an aggressive pro-inflammatory or “M1” phenotype to a pro-healing or “M2” phenotype, depending on the wound healing stage, to ensure proper healing. Because dysregulated macrophage responses have been linked to impaired healing of diabetic wounds, macrophages are being considered as a therapeutic target for improved wound healing. In this review, we first discuss the role of macrophages in a normal skin wound healing process and discuss the aberrations that occur in macrophages under diabetic conditions. Next we provide an overview of recent macrophage-based therapeutic approaches, including delivery of ex-vivo-activated macrophages and delivery of pharmacological strategies aimed at eliminating or re-educating local skin macrophages. In particular, we focus on strategies to silence key regulator genes to repolarize wound macrophages to the M2 phenotype, and we provide a discussion of their potential future clinical translation.
Keywords: macrophage, wound healing, inflammation, M1, M2, IRF5, siRNA, drug delivery
Graphical abstract
A dysregulated macrophage phenotype changing from pro-inflammatory to pro-healing has been linked to impaired healing of diabetic wounds. Sharifiaghdam et al. discuss the current macrophage-based therapeutic strategies in two main categories: pharmacological approaches to influence macrophages directly at the wound site and transplantation of ex-vivo-activated macrophages.
Overview of wound repair
The skin is the first line of protection against chemical and physical assault, pathogen invasion, and prevention of unregulated dehydration. At the same time, it is involved in the transmission of sensory information and has a significant role in maintaining homeostasis.1 When the integrity of the skin is compromised, it can affect the normal function of many cells, including endothelial cells, fibroblasts, and keratinocytes in each layer of the skin.2 Therefore, proper recovery of the skin by wound healing is one of the most complex and tightly regulated processes involving evolutionarily developed genetic, epigenetic, and molecular processes to accomplish the goal of reorganizing different cells in space and time.3 This process occurs in four overlapping phases: hemostasis, inflammation, proliferation, and remodeling.4,5 A considerable amount of evidence has positioned macrophages as key regulators of these steps, playing an essential role during the different healing phases by readily adapting their phenotype according to spatiotemporal cues they receive during each phase.6 In general, these phenotypes have been differentiated into pro-inflammatory M1-like macrophages (M1) on the one hand and pro-healing/anti-inflammatory M2-like macrophages (M2) on the other hand.7
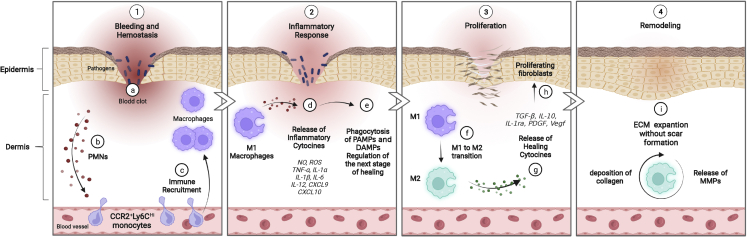
An overview of the healing process is presented in Figure 1. Upon injury, the accumulated blood plug at the site of skin injury promotes the initial invasion of immune cells, specifically polymorphonuclear leukocytes (PMNs).3,8 The arrival of these cells characterizes the beginning of the inflammatory phase.9 This rapid, initial influx of PMNs is followed by successive waves of infiltrating monocytes that, in response to wound microenvironmental cues (e.g., low oxygen levels, microbial products, damaged cells, and activated lymphocytes), differentiate into M1-type macrophages for scavenging, phagocytosis, and antigen presentation during the inflammatory phase of healing.10 When the acute injury is under control, macrophage gene expression gradually shifts from M1 (pro-inflammatory) macrophages to an M2 (pro-healing) phenotype with the capability for recruitment of stem cells and secretion of cytokines and growth factors that stimulate keratinocytes, fibroblasts, and endothelial cells to proliferate, differentiate, and migrate.11 Through this plasticity, a positive feedback loop is established in which increasing normalization of the tissue microenvironment drives a progressively more pro-healing role for macrophages, which, in turn, promotes tissue repair.12, 13, 14 This process also involves massive angiogenesis, which is needed for the supply of oxygen and nutrients necessary for fibroblasts and other cells to reconstitute the tissue matrix in the remodeling phase.15, 16, 17, 18, 19
Figure 1.
The role of macrophage cells in wound healing processes
(1) a–c: hemostasis phase. An initial influx of polymorphonuclear leukocytes (PMNs) in the blood clot is followed by successive waves of infiltrating monocytes that differentiate into macrophages in the wound. Infiltration of immune cells characterizes the beginning of the inflammatory phase. (2) d and e: during this phase, the differentiated M1 macrophages clear pathogens and cell debris, like pathogen-associated modifying proteins (PAMPs) and damage-associated modifying proteins (DAMPs), and release nitric oxide (NO), reactive oxygen species (ROS), and pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1α (IL-1α, IL-1β, IL-6, IL-12, CXCL9, and CXCL10, to prepare the wound bed for the next stage. (3) f–h: during the proliferation phase, the macrophage phenotype changes from an inflammatory to a healing state, releasing healing cytokines, such as transforming growth factor β (TGF-β), IL-10, IL-1 receptor antagonist (IL-1ra), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and vascular endothelial growth factor (Vegf). Proliferation, differentiation, and migration of keratinocytes, fibroblasts, and endothelial cells also take place. (4) i: the remodeling phase is the last stage of wound healing, in which macrophages still play a key role by releasing matrix metalloproteinases (MMPs) to the deposited ECM to restore tissue strength with minimal scar formation. Created with BioRender.
The importance of macrophages in coordination of the wound healing response becomes apparent when their activation is disturbed. A study using genetically modified LysMCre/DTR mice, which allow inducible depletion of macrophages by diphtheria toxin (DT) administration, provided strong evidence that these cells are required for normal wound healing, including promoting angiogenesis, collagen deposition, and wound closure.20 Other studies have found that properly regulated numbers and phenotypes of macrophages are crucial for efficient wound repair and that dysregulation of either of these may lead to chronic skin inflammation and impaired wound healing.21,22 A particular case is chronic wounds, which are defined as wounds stalled in a constant and excessive inflammatory state with increased numbers of M1 macrophages, inactivated growth factors, increased oxidative damage, and deficient local angiogenesis, which prevents the normal tissue repair program to be executed.23,24 For example, the hyperglycemic environment in diabetic wounds leads to high reactive oxygen species (ROS) levels and stimulation of the macrophages to secrete pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6, which encourages the vicious cycle of maintaining M1 macrophage polarization and chronic inflammation.
Because of a better understanding of the crucial role of macrophages in the different phases of the wound healing process, shifting the macrophage phenotype from the pro-inflammatory M1 to the pro-healing M2 phenotype has become an active area of research to promote wound healing25 and diabetic wound healing in particular.26,27 The objective of this review is to discuss the current macrophage-based therapeutic strategies in diabetic wound healing. We first go more deeply into the origin of skin macrophages and their plasticity, flexibility, and heterogeneity under physiological and diabetic wound conditions. Then we provide an overview of current developments of wound healing strategies controlling macrophage function or phenotype to accelerate wound healing.
Origin and role of macrophages in skin wounds
Macrophages in skin wounds originate from two primary sources: tissue-resident macrophages (Langerhans cells and dermal macrophages), stemming from yolk sac hematopoietic tissue in the uterus,28 and macrophages that are generated from blood monocytes (recruited macrophages).29 The major function of resident macrophages is maintenance of skin homeostasis and integrity.30 The development of resident macrophages requires colony-stimulating factor 1 (CSF1; also known as macrophage colony-stimulating factor [M-CSF]).31,32 After an injury, these cells are likely early responders, leading to subsequent recruitment of CCR2+Ly6CHi blood monocytes into the wound.33, 34, 35 These recruited monocytes differentiate into Ly6CHiF4/80+ macrophages in the inflammatory phase of wound healing and later switch to Ly6CLoF4/80+ macrophages to accelerate wound healing.33,36,37
The first phase after injury is hemostasis, in which platelets accumulate at the site of tissue injury to form a blood plug.8 The initial plug, which is stabilized by fibrin fibers, can act as a matrix for the various infiltrating cells.3 The interactions of coagulant factors, along with activation of the endothelium, promote the initial invasion of immune cells, specifically PMNs. The arrival of these cells characterizes the beginning of the inflammatory phase (Figures 1A–1C).9
As mentioned previously, this rapid, initial influx of PMNs is followed by successive waves of infiltrating monocytes that differentiate within the wound into macrophages. During this step, to accommodate recruitment of a significant number of monocyte cells from the blood, there is an increase in myeloid-lineage-committed multipotent progenitors and monocytes in the bone marrow that results in a 70% increase in monocytes in the blood circulation on day 2.38 After recruitment in the wound, these monocytes differentiate into activated M1 macrophages with an inflammatory phenotype.39 These macrophages can recognize pathogen-associated modifying proteins (PAMPs) on the surfaces of bacteria or fungi and damage-associated modifying proteins (DAMPs), like extracellular DNA, RNA, and ATP, which are released because of cell death.40,41 M1 macrophages produce nitric oxide (NO), ROS,42 and pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-12 (Figures 1D and 1E), C-X-C motif chemokine ligand 9 (CXCL9), and CXCL10,40 to clear pathogens and cell debris and stimulate proliferation of wound cells, such as fibroblasts and keratinocytes, to be able to regulate the next stage in the repair process.43
In vitro studies have shown that, after activation by lipopolysaccharide (LPS) and cytokines, like interferon gamma (IFN-γ) and TNF-α, macrophages become polarized into M1 macrophages, as characterized by Toll-like receptor (TLR)-2, TLR-4, CD80, CD86, inducible nitric oxide synthase (iNOS), and major histocompatibility complex (MHC) class II surface phenotypes. M1 macrophages release various cytokines and chemokines (such as TNF-α, IL-1α, IL-1β, IL-6, IL-12, CXCL9, and CXCL10) that exert positive feedback on unpolarized macrophages to change to the M1 state. Key transcription factors, such as nuclear factor κB (NF-κB), STAT1, STAT5, IRF3, and IRF5, have been shown to regulate expression of M1 genes.42,44
Transition from the inflammation phase to the proliferation phase is characterized by repolarization of M1 macrophages to the M2 phenotype.3,45,46 The M2 phenotype is known to be a healing-associated macrophage with downregulated inflammatory factors and ROS levels and upregulated anti-inflammatory cytokines (e.g., TGF-β, IL-10, IL-1 receptor antagonist [IL-1ra], and the IL-1 type II decoy receptor), growth factors (e.g., platelet-derived growth factor [PDGF], epidermal growth factor, and vascular endothelial growth factor [Vegf]). The M2 phenotype promotes angiogenesis as well as proliferation, migration, and differentiation of fibroblasts, as required for tissue repair47, 48, 49 (Figures 1F–1H). M2 macrophages are also involved in recruitment of stem cells to wounds, an important driving force in tissue repair.50 M2 polarization occurs in response to downstream signals of cytokines, such as IL-4, IL-13, IL-10, IL-33, and TGF-β.51 M2 macrophages can be identified by their expression of surface markers, such as the mannitol receptor, CD206, CD163, CD209, FIZZ1, and Ym1/2, and up-regulation of cytokines and chemokines, such as IL-10, TGF-β, CCL1, CCL17, CCL18, CCL22, and CCL24.42,44,52
During the final remodeling phase, fibroblasts and other cells reconstitute the tissue matrix via deposition of collagen type III and, later on, collagen type I,16 and the number of macrophages starts to decline, but the remaining macrophages still play a role by releasing matrix metalloproteinases (MMPs) (Figure 1I).53, 54, 55 MMPs are major proteolytic enzymes involved in turnover of the extracellular matrix (ECM) during cutaneous wound repair and restoration of skin functionality with minimal scar formation.56 Impaired ECM turnover results in an abnormal remodeling phase that leads to unregulated cell proliferation as well as impaired apoptosis, cell differentiation, and physiological functions of the skin with excessive scar formation.57, 58, 59
Macrophage dysregulation in diabetic wounds
In the early stages of repair, around 85% of macrophages in the wound have the M1 pro-inflammatory phenotype, which switches to around 80%–85% anti-inflammatory M2 macrophages by days 5–7.60 Studies have shown that pre-injury depletion of macrophages in mouse models causes defects in re-epithelization, formation of granulation tissue, angiogenesis, wound cytokine production, and myofibroblast-associated wound contraction.20,61,62 Macrophage depletion during formation of granulation tissue, about 3 days after injury, is also associated with vascularization defects, delay in wound closure, and granulation tissue maturation.62 Another study of isolated macrophages during the wound healing process on days 5, 10, and 20 after injury in wild-type mice indicated that pro-inflammatory macrophages were detectable until day 5. These macrophages expressed high levels of IL-1β, MMP-9, and NO synthase (NOS). By day 10, the expression of these pro-inflammatory factors decreased, concurrent with an increase in expression of the anti-inflammatory markers CD206 and CD36 and growth factors insulin growth factor (IGF)-1, TGF-β, and Vegf with efficient wound repair. Wound closure was completed after 20 days, when elevated expression for CD206, CD36, and TGF-β was still detectable, but not of IGF-1 or Vegf.63
More and more evidence is accumulating, and the pivotal role of macrophage phenotype switching is emerging as a key process to achieve successful wound healing. Any perturbation of this process could result in stalling of the repair process in the inflammatory phase, leading to a delay in wound closure and increased risk of infection.23 Pathological conditions that can induce such perturbations include aging, obesity, infection, and diabetes.
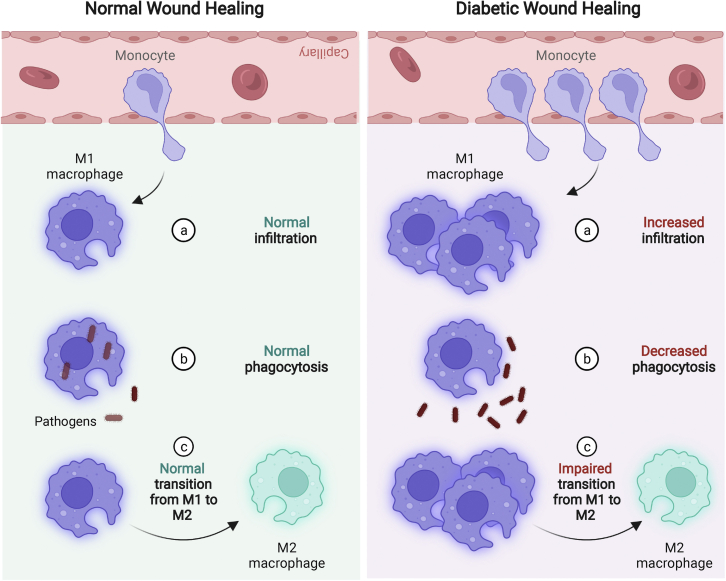
Diabetes is a major and increasing health problem that leads to hyperglycemia as a consequence of cellular insulin resistance and insulin deficiency.64 An elevated blood glucose level has a significant effect on the immune system and could lead to alterations in the number and phenotype of wound-associated macrophages. It has been reported that the diabetic microenvironment leads to increased myelopoiesis in hematopoietic stem cells, which results in elevated levels of circulating blood monocytes (Ly6CHi), which enter wounds and differentiate into macrophages with characteristic M1-like signatures (IL-1β, TNF-α).65 This finding was supported by a recent study reporting that the number of infiltrating monocytes is higher in wounds of type 2 diabetic (T2D) mice compared with healthy mice22 (Figure 2A). In addition, it was found that wound-derived macrophages in diabetic mice showed a lack of phenotypic switching to the M2 phenotype. The hyperglycemic condition in diabetic wounds decreases the inflammation-resolving functions of macrophages, such as their phagocytic activity (Figure 2B). Phagocytosis plays a role in the switch of the wound macrophage phenotype from M1 to pro-healing M2 (Figure 2C) and in release of IL-10, TGF-β, and all pro-healing factors.60,63 Increased pro-inflammatory signals in diabetic wounds amplify the pro-inflammatory effect of M1 macrophages, which continue to release inflammatory cytokines, proteases, and ROS, leading to degradation of the ECM. This phenotypic imbalance of macrophages causes a persistent state of inflammation in the diabetic wound that hinders the pro-healing activity of endothelial cells, keratinocytes, and fibroblasts.60
Figure 2.
Macrophage dysregulation in diabetic wounds leads to delayed wound healing
Compared with normal wounds, increased numbers of infiltrated BM-produced monocytes are present in diabetic wounds, which, in turn, leads to a higher number of macrophages (a). The capability of M1 macrophages to phagocytose pathogens is decreased in diabetic wounds (b). Macrophage transition from M1- to M2-like phenotypes is impaired in diabetic wounds, leading to an accumulation of inflammatory M1 macrophages (c). M1, macrophage with a pro-inflammatory phenotype; M2, macrophage with a pro-healing phenotype. Created with BioRender.
Diabetic foot ulcers (DFUs) are a very severe and common complication in diabetic patients and are the reason for approximately 80% of lower limb amputations.66 In spite of many advances in wound healing, such as using growth factors in wound dressings, hyperbaric oxygen therapy, and negative pressure, complete healing of DFUs remains challenging. The growing number of amputations because of DFUs underscores the importance of continued investigation in this field.67,68 Strategies that target macrophages as key regulators of wound inflammation and healing are considered promising new therapeutic approaches. In the next sections, we discuss these different approaches in the context of wounds, in particular diabetic wounds.
Macrophage-based therapies
Given the role of macrophages in the progression of wound repair, macrophage-based therapies aim to inhibit inflammation or enhance pro-healing anti-inflammatory activities. The therapeutic strategies can be divided into 2 main categories: pharmacological approaches to influence macrophage phenotype or products directly in the wound, and transplantation of ex-vivo-activated macrophages. Both therapeutic strategies are discussed below.
Pharmacological approaches to influence macrophage phenotype or products
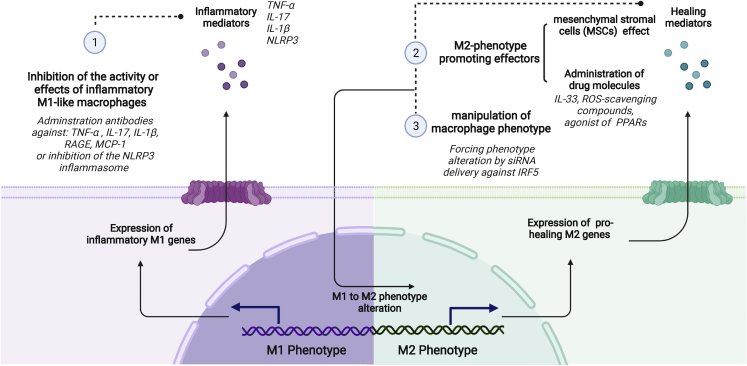
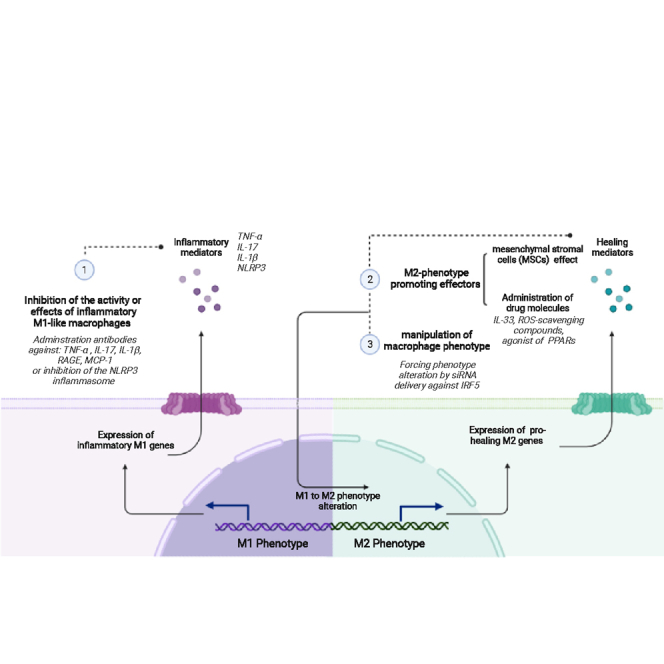
Pharmacological strategies to modulate the macrophage phenotype or products in wounds can be divided into the following general categories: inhibition of the activity or effects of inflammatory M1-like macrophages, M2 phenotype promoting strategies, and manipulation of macrophage phenotype. They are discussed below (Figure 3).
Figure 3.
Pharmacological approaches to influence macrophage phenotype or activity
(1) Inhibition of the activity or effects of inflammatory M1-like macrophages can be achieved by administration of therapeutic antibodies that inhibit effects of inflammatory mediators like TNF-α, IL-1β, IL-17, advanced glycosylation end products (AGEs), and the monocyte chemoattractant protein 1 (MCP-1) or Nod-like receptor protein 3 (NLRP3) inflammasome. (2) M2 phenotype-promoting strategies include use of mesenchymal stromal cells (MSCs) to induce anti-inflammatory M2 like characteristics in macrophages or administration of drugs like IL-33, ROS-scavenging compounds, and agonist of peroxisome proliferator-activated receptors (PPARs) to promote the M2 phenotype. (3) Manipulation of macrophage phenotype can be achieved by delivery of siRNA against interferon regulatory factor 5 (IRF5), which enforces phenotype alteration. Created with BioRender.
Inhibition of the activity or effects of inflammatory M1-like macrophages
In all chronic wounds, despite their different etiologies, macrophages tend to get stuck in an M1-like phenotype, leading to increased levels of pro-inflammatory mediators like TNF-α.69,70 This, in turn, leads to recruitment of more immune cells and also affects MMPs, leading to destruction of the ECM scaffold cells require to repopulate and close the wound.71 It has been demonstrated that administration of therapeutic antibodies that inhibit TNF-α (e.g., infliximab topically or adalimumab subcutaneously) accelerates wound closure in a diabetic mouse model64 and considerably improved wound repair in chronic venous ulcers (CVUs) of individuals whose wounds failed to respond to any previous conventional treatment.69,72 In addition, using a secretory leukocyte protease inhibitor (SLPI)-deficient null mouse model with an imbalanced M1/M2 macrophage phenotype, it was found that neutralizing TNF-α at wound sites could accelerate the rate of healing macroscopically (Figure S1A, i and ii) and microscopically (Figure S1A, iii) by altering the balance between M1 and M2 macrophages on day 3 in a dose-dependent manner.69
There is compelling evidence that accumulation of advanced glycosylation end products (AGEs) in diabetic wounds, a common consequence of persistently elevated blood glucose, contribute significantly to the pathology associated with impaired wound healing.73 It has been shown that M1 macrophages express high numbers of the receptor for AGEs (RAGE) under the diabetic condition and that AGE-RAGE signaling underlies the impaired phagocytosis of macrophages. As mentioned before, there is evidence to suggest that the switch from M1 to M2 phenotype may in part be regulated by phagocytosis of apoptotic cells.25 In vitro work has shown that dysregulated phagocytosis by macrophages is followed by a decrease in expression of pro-healing factors, such as TGF-β1, which is believed to contribute to the observed subsequent disruption of macrophage phenotype switching.74 The disrupted phenotype transition negatively affects wound repair through development of chronic inflammation.75 Topical administration of anti-RAGE antibodies to full-thickness excisional wounds in diabetic mice results in an increase in phagocytosis of M1 macrophages, which has been associated with a gradual transition to the M2 phenotype and improved wound healing.76
In diabetic wounds, in which IL-1β, an inflammatory cytokine, is upregulated, it was found that inhibiting the IL-1β pathway with a neutralizing antibody induced the switch from pro-inflammatory to healing-associated macrophages and improved healing of these wounds.77 Figure S1B, i, shows hematoxylin-and-eosin-stained cryosections of skin tissue on day 10 after injury. The wounds treated with antibodies against IL-1β significantly increased re-epithelialization and granulation tissue (gt) in IL-1β antibody (IL1ab)-treated samples compared with control immunoglobulin G (IgG)-treated samples (Figure S1B, ii and iii). Similarly, local administration of a blocking antibody against IL-17 on days 0, 1, and 2 upon wounding inhibited M1-like macrophage activity and significantly accelerated wound closure compared with the untreated group during 10 days of monitoring of diabetic mice.78
It has also been shown that overactivation of the Nod-like receptor protein 3 (NLRP3) inflammasome pathway in macrophages contributes to development of diabetic complications,79 including delayed diabetic wound healing and severe cardiac dysfunction.80,81 Therefore, inhibition of the NLRP3 inflammasome pathway was explored by local application of calcitriol (1,25-dihydroxyvitamin D3), which was proven to promote diabetic skin wound healing.82,83 A later study indicated that topical application of calcitriol promotes corneal wound healing and reinnervation in diabetic mice by inhibiting activation of the NLRP3 inflammasome.84 These data suggest that use of specific molecules that can inhibit the activity or effects of the pro-inflammatory M1 phenotype could be a rational approach to reduce excessive inflammation and expedite wound healing.
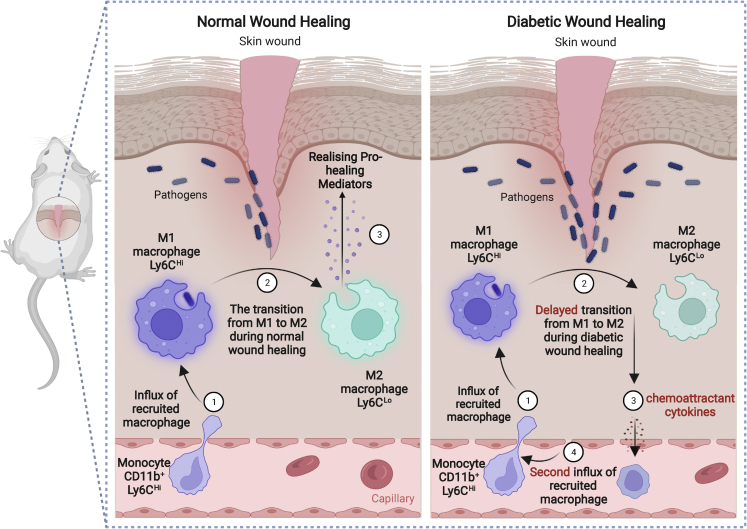
As explained earlier, infiltrating blood monocyte-derived M1 macrophages (Ly6CHi) are critical for the initial inflammatory phase and, under normal wound conditions, switch to Ly6CLo M2 cells. It was shown that, in diabetic wounds, not only is the Ly6CHi-to-Ly6CLo phenotype transition is delayed, but there is also a second influx of Ly6CHi monocytes/macrophages during the reparative phase that results in significantly higher numbers of M1 inflammatory macrophages and elevated levels of pro-inflammatory factors (Figure 4).60 It was also found that the second wave of Ly6CHi cells in diabetic wounds corresponded to a spike in MCP-1 (monocyte chemoattractant protein-1). Intraperitoneal injection of anti-MCP-1 to block the second influx of Ly6CHi monocyte/macrophages in diabetic mice 24 h before the observed second wave of Ly6CHi monocyte/macrophages resulted in a significant improvement in wound healing compared with normal rabbit-serum-injected controls.60 This study suggests that time-dependent inhibition of M1 macrophage accumulation may be a novel therapeutic strategy for impaired diabetic wound healing.
Figure 4.
The influx of CD11b+Ly6CHi monocytes/macrophages during wound healing under normal and diabetic conditions
During normal wound healing, monocyte-derived Ly6CHi macrophages are present within 48 h of injury and rapidly transition to Ly6CLo macrophages, releasing pro-healing mediators at the wound site. In diabetic wounds, transition of Ly6CHi to Ly6CLo macrophages is delayed, leading to a second influx of Ly6CHi monocyte/macrophages during the reparative phase. Created with BioRender.
M2-promoting strategies
Besides inhibiting the consequences of M1 macrophage activity, it is also possible to induce a shift toward the M2 phenotype by administration of M2-promoting cells or effector molecules to enhance tissue repair. This approach is discussed next.
MSC-educated M2 macrophages
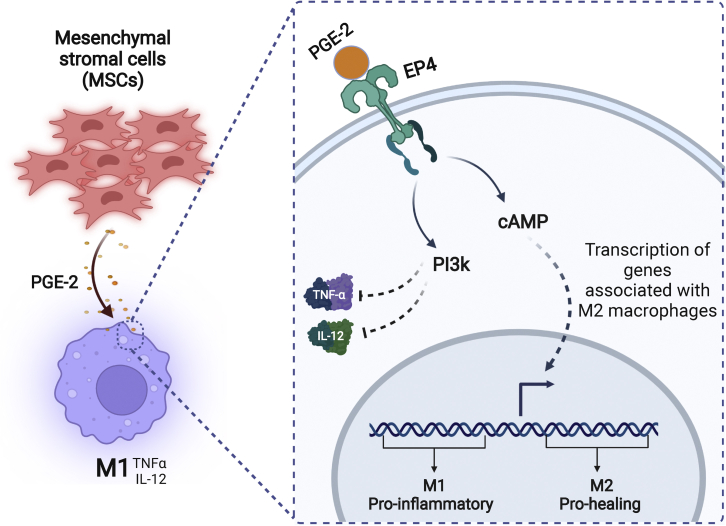
M2-promoting effector molecules can be secreted by other cell types that act as “M2 educators.” Evidence has shown that mesenchymal stromal cells (MSCs), fibroblast-like cells originally isolated from bone marrow (BM), can secrete many growth factors with powerful modulating effects on macrophages with a direct effect on wound healing.85 One in vitro study showed that, when cocultured with MSCs, macrophages acquired anti-inflammatory M2-like characteristics (low production of pro-inflammatory cytokines, such as IL-12 and TNF-α, and high production of pro-healing cytokines, such as IL-10).86 It was found that MSCs secrete an important regulator, prostaglandin E-2 (PGE-2),87,88 which is able to bind to the EP4 receptor on M1 macrophages and activate two independent pathways: (1) the phosphatidylinositol 3-kinase (PI3K) pathway, which inhibits pro-inflammatory cytokines such as TNF-α and IL12, and (2) the cyclic adenosine monophosphate (cAMP) pathway, which leads to transcription of genes that can induce up-regulation of M2-like markers (Figure 5).89 These results were confirmed in vivo in a cutaneous wound model, showing that MSCs injected systemically into mice 1 day after full-thickness skin excision could concentrate at the wound site and promote the shift of M1 toward M2, resulting in better wound healing.90
Figure 5.
MSC-educated M2 macrophages
Prostaglandin E-2 (PGE-2), secreted from MSCs, can bind to EP4 receptors on M1 macrophages at the wound site and repolarize them to pro-healing M2 macrophages via two independent pathways: (1) the phosphatidylinositol 3-kinase (PI3K) pathway, which inhibits TNF-α and IL-12, and (2) the cyclic adenosine monophosphate (cAMP) pathway, which leads to activation of transcription of genes associated with the M2 macrophage phenotype. Created with BioRender.
Induction of M2 macrophages by the administration of drugs
ILs are a first example of therapeutic molecules that can be used to repolarize M1 macrophages into M2. For example, administration of IL-33, a member of the IL-1 family that potently drives production of T helper 2-associated cytokines, has shown beneficial effects in treatment of liver ischemia91 and atherosclerosis.92 It has been shown that intraperitoneal injection of murine recombinant IL-33 into mice subjected to incisional skin wounds once a day from day 0–3 can shift macrophages from M1 to M2, leading to accelerated collagen accumulation, wound re-epithelialization, and wound healing.93
IL-25, an IL-17 cytokine family member, has been shown to have an inhibitory effect on diabetic wounds, inhibiting the inflammatory response and promoting tissue remodeling.94 It has been reported that local intracutaneous injection of IL-25 into a full-thickness wound of diabetic mice can promote polarization of wound macrophages to M2 through the PI3K/Akt (Ak strain transforming) and mammalian target of rapamycin (mTOR) signaling pathways with a subsequent release of pro-healing cytokines at the wound site. The study showed that M2-polarized macrophages can accelerate the proliferation and migration behavior of fibroblasts in a paracrine manner.95
Another example of drug molecules used for macrophage repolarization are ROS-scavenging compounds. It has been shown that ROS production in the wound is usually associated with activation of M1 rather than M2 macrophages.96 Therefore, administration of ROS-scavenging compounds, like natural or chemical antioxidants, at the wound site can promote wound closure by repolarizing macrophages to the M2 phenotype.97 In another study, a type of ROS-scavenging hydrogel was developed, consisting of polyvinyl alcohol (PVA) cross-linked by a ROS-responsive linker.98 The hydrogel acted as an effective ROS scavenger, promoting wound closure by decreasing ROS levels and up-regulating M2-phenotype macrophages.98
A final example of M2 promoting drug molecules are the natural agonists (e.g., omega-3 fatty acids) or synthetic agonists (e.g., clofibrate, fenofibrate, and bezafibrate) of the peroxisome proliferator-activated receptors (PPARs), consisting of three different isotypes: PPAR-α, PPAR-δ (PPAR-β), and PPAR-γ. They are interesting tools for pharmacological intervention to treat inflammatory conditions. PPAR-γ is a member of the superfamily of nuclear receptors,99 and it functions as a ligand-activated transcription factor with pleiotropic effects on inflammation.100 A study clarified the relationship between PPAR-γ activation and innate immune cells, such as macrophages.101 The study suggested that macrophages are one of the main cellular targets for PPAR-γ activation and that its anti-inflammatory features are related to macrophage M2 polarization, reducing inflammation and promoting a return to homeostasis.101 Previous work has shown that activated M2 macrophages express significant PPAR-γ levels102 and that reducing its levels could result in overactivation of the immune system and elevated inflammatory responses.103 Figure S2A shows delayed wound closing in PPARγ knockout (KO) mice in comparison with control wild-type (WT) mice during 12 days of monitoring after wounding.104 Local administration of PPAR agonists reduced the oxidative wound microenvironment105 and promoted a healing-associated macrophage phenotype, leading to improved wound healing.106,107 Other studies support PPARβ/δ as a valuable pharmacologic wound healing target in macrophages. Figures S2B–S2F show the results of topical application of the polymer-encapsulated PPARβ/δ agonist GW501516 (GW) to full-thickness excisional splint wounds on the dorsal skin of diabetic mice.105 Providing an earlier and sustained dose of GW to the wound by encapsulating GW in core-shell poly(L-lactide) and poly(D,L-lactide-co-glycolide) (PLLA:PLGA:GW) microparticles significantly reduced ROS levels close to those of WT wounds, whereas ROS levels were significantly higher in the control group treated with microparticles without encapsulated GW (PLLA:PLGA:veh [vehicle]) (Figure S2B). Treatment with PLLA:PLGA:GW accelerated diabetic wound closure (closed within 7 days) (Figures S2C and S2D), with longer and thicker wound epithelium formation (Figures S2E and S2F), suggesting that it is an appealing strategy for down-regulating inflammation and improving healing of chronic wounds.
Manipulation of macrophage phenotype
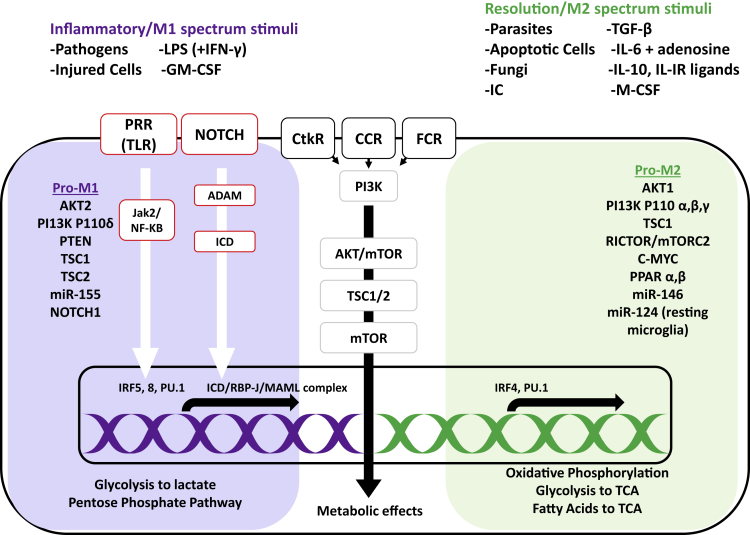
Analysis of the differential gene expression of M1 and M2 macrophages yielded a list of genes with mRNA levels differing greatly between both phenotypes.108 A summary of molecular pathways involved in M1 and M2 macrophages is depicted in Figure 6. Of particular interest is the PI3K/AKT pathway, which mediates an inflammatory M1 type signal through tuberous sclerosis complex (TSC)/mTOR (tuberous sclerosis/mTOR) mediators.109 Another frequent signaling pathway involved in the M1 phenotype is NOTCH1.110 In response to inflammatory cues such as LPS, IFN-γ, or IL-1β, macrophages increase expression of NOTCH receptors that promotes M1 gene expression via complexation of intracellular domain (ICD) and recombination signal binding protein for immunoglobulin kappa J region (RBP-J) complex and recruitment of the mastermind-like (MAML) coactivator.111,112 Pathogen recognition receptors (PRR) such as TLR are another signaling pathway that promotes inflammatory gene expression in macrophages via Janus-activated kinase 2 (Jak2) and NF-κB activation.113 It was discovered that IFN regulatory factors (IRFs) are the intracellular signaling elements that determine gene transcription of macrophage cells in response to initial signal sensing by PRRs.114 IRFs are responsible for mediating PRR signals to chromatin and for finally determining macrophage phenotype.115 There are nine IRF family members: IRF1, IRF2, IRF3, IRF4, IRF5, IRF6, IRF7, IRF8/ICSBP (interferon consensus sequence binding protein), and IRF9.116 Studies so far have shown that IRF5 and IRF8 are involved in promotion of M1 macrophage activation (M1), whereas IRF4 promotes inflammatory M2 phenotype activation.116
Figure 6.
Key stimuli and molecular pathways in macrophages with the inflammatory (M1) versus healing phenotype (M2)
The M1 phenotype is activated by signals from pathogens, injured cells, and in vitro stimuli, and the M2 phenotype is activated by parasites, fungi, apoptotic cells, immune complexes, and other cytokine/growth factor stimuli. Signals that are sensed via pathogen-recognition receptors (PRRs), such as Toll-like receptors (TLRs), result in Janus-activated kinase (Jak) 2 and nuclear factor κB (NF-κB) activation. Signals received via Notch receptors, cytokine receptor (CtkR), chemokine receptor (CCR), and Fc receptor (FcR) stimulation also define gene expression and downstream metabolic reprogramming. IRF5 and IRF8 induce inflammatory M1 gene expression, whereas IRF 4 promotes M2 phenotype genes that, in turn, promote changes in nutrient uptake and metabolic pathways, depending on which part of the gene is expressed. LPS, lipopolysaccharide; GM-CSF, granulocyte monocyte colony-stimulating factor; IC, immune complex; M-CSF, monocyte colony-stimulating factor; PI3K, phosphatidylinositol 3-kinase; AKT, serine threonine kinase; mTOR, mammalian target of rapamycin; PTEN, phosphatase and tensin homolog; TSC, tuberous sclerosis complex; PPAR, peroxisome proliferator-activated receptor; ADAM, a disintegrin and metalloproteinase; RBP-J, recombination signal binding protein for immunoglobulin κJ region; MAML, mastermind-like; Rictor, rapamycin-insensitive companion of mTOR; TCA, tricarboxylic acid/Krebs cycle. Adapted from Amici et al.113
These identified genes form potential targets for alteration by gene editing tools. Arguably the most attractive approach to modify macrophage polarization is to reduce inflammatory gene expression through RNA interference (RNAi) because multiple genes can be downregulated simultaneously. Small interfering RNAs (siRNAs) are a class of short double-stranded RNA molecules (21–25 nt in length) that induce gene silencing by targeting complementary mRNA strands for degradation.117 Unlike conventional drugs or small molecules, which have a limited range of protein targets, siRNA may be used to interfere with the expression of nearly any gene transcript in a specific manner. Despite the fact that siRNAs are capable of knocking down targets in various diseases, clinical use is hampered by the need for safe and effective delivery systems.118,119 Naked siRNA double strands are relatively unstable in blood and serum, being prone to rapid degradation by endo- and exonucleases. Because of to its large molecular weight (13 kDa) and polyanionic nature (negative charges), a naked siRNA double strand does not freely cross the cell plasma membrane.120 In this regard, specific formulations, such as non-viral delivery vectors, can contribute to enhanced siRNA delivery to target cells and improve siRNA stability.121 For example, siRNA silencing of the genes responsible for production of TNF-α in macrophages was investigated to improve diabetic wound healing.122, 123, 124 Chronic upregulation of TNF-α in DFUs leads to overproduced MCP-1/CCL2 in fibroblast cells, which helps with recruitment of additional macrophages to the wound bed and contributes to the positive feedback cycle of inflammation.125It has been demonstrated that in vitro silencing of TNF-α in macrophages co-cultured with fibroblasts (using lipidoid nanoparticles [LNPs] loaded with siTNF-α) leads to downregulation of MCP-1, putting an end to the paracrine feedback loop between TNF-α and MCP-1.122 Later, it was shown that in vivo application of siTNF-α-loaded LNPs to diabetic wounds also reduces TNF-α expression to a similar level as in normoglycemic mice, reduces the inflammatory response, and reduces the wound area and healing time.124 In addition, siRNA silencing of MMP-9 was tried, another secreted factor that leads to breakdown of the local ECM and delayed wound healing.126, 127, 128, 129, 130, 131, 132 A recent study used a collagen/glycosaminoglycan scaffold to deliver siMMP-9 in a DFU wound model.132 MMP-9 gene expression levels in M1 macrophages could be efficiently reduced by 50% in the siMMP-9-treated group compared with the non-transfected group.132Topical administration of siMMP-9-loaded nanoparticles (NPs) around the wound bed of diabetic rats was investigated, showing a significant reduction in expression of MMP-9 and improved wound healing.126,128 However, because repeated injections were required to sustain long-term silencing of MMP-9, in a follow-up study, siMMP-9 was loaded in a wound dressing for sustained siRNA release.130 A hybrid hydrogel dressing was developed for localized prolonged delivery of siMMP-9, achieving 65% release over 7 days.127 A summary of the main studies of diabetic wound healing by siRNA silencing can be found in Table 1, from which it is apparent that NPs and scaffolds have mostly been used as delivery systems.
Table 1.
Reported studies on diabetic wound healing with siRNA targeted against mediators like; Keap1, MMP2, MMP9, TNF alpha, GM3s, PHD2, HIF-1, and IRF5
| Target mRNA | Wound | Study model | Delivery system | Results | Reference |
|---|---|---|---|---|---|
| MMP-2 | 9-mm full-thickness skin wound on the dorsal area of STZ-induced diabetic mice | • in vitro • in vivo Female C57BL/6 mice |
MMP-2 siRNA was chemically tethered to the end of multi-armed PEG and subsequently clustered to submicron particles complexed with linear PEI. MMP-cleavable peptide was used as a linker between PEG and siRNA for stimulus-responsive release of siRNA depending on local MMP concentration. | • downregulated MMP-2 gene expression • long-term MMP-2 gene silencing • Accelerated wound closure |
133 |
| MMP-9 | 10-mm full-thickness skin wound on the dorsal area of STZ-induced diabetic rats | • in vitro • in vivo Sprague-Dawley (SD) rats (6 weeks, male) |
siMMP-9 complexed with Gly-triethylenetetramine (GT) and then loaded into a thermosensitive hydrogel dressing | • downregulated MMP-9 gene expression • biocompatible hydrogel dressing • long-term release of GT/siMMP-9 for up to 7 days • enhanced diabetic wound healing |
127 |
| 10-mm full-thickness skin wound on the dorsal area of STZ-induced diabetic rats | • in vitro • in vivo Sprague-Dawley (SD) rats (5–6 weeks, male) |
MMP-9 siRNA loaded in wound dressing contains hyperbranched cationic polysaccharide (HCP) derivatives. | • downregulated MMP-9 gene expression • biocompatible dressing • enhanced diabetic wound healing |
131 | |
| 10-mm full-thickness skin wound on the dorsal area of STZ-induced diabetic rats | • in vitro • in vivo SD rats (male) |
MMP-9 siRNA encapsulated in a dressing containing bacterial cellulose (BCP)-HCP | • downregulated MMP-9 gene expression • long-term release of MMP-9 siRNA • enhanced diabetic wound healing |
130 | |
| full-thickness skin wound on the dorsal area of STZ-induced diabetic rats | • in vitro • in vivo SD rats |
Star-branched cationic polymer β-CD-(D3)7, which consists of β-cyclodextrin and third-generation poly(amidoamine) (PAMAM) was used as a carrier to take MMP-9 siRNA. | • downregulated MMP-9 gene expression • enhanced diabetic wound healing • decreased infiltration of inflammatory cells around the local wound |
128 | |
| DFU-mimicking conditions | • in vitro | MMP-9 siRNA loaded in collagen/glycosaminoglycan (Col/GAG) scaffolds. | • downregulated MMP-9 gene expression in fibroblast and macrophage cells | 132 | |
| Keap1 | 10-mm full-thickness skin wound on the dorsal area of diabetic mice from The Jackson Laboratory (Bar Harbor, ME). | • in vitro • in vivo male diabetic Lepr db/db mice, aged 12 weeks |
liposome and protein hybrid NP delivery system | • downregulated Keap1 gene expression • increased transfection efficacy Keap1 siRNA • accelerated diabetic tissue regeneration |
134 |
| TNF-α | co-culture model of fibroblast and macrophage cells | • in vitro | LNPs to deliver TNF-α siRNA | • downregulated TNF-α gene expression • decreased production of monocyte chemotactant protein 1 (MCP-1/CCL2) to inhibit the recruitment of additional macrophages to the wound site |
122 |
| 8-mm full-thickness skin wound on the right and left flanks | • in vitro • in vivo genetically diabetic BKS.Cg-Dock7m +/+ Lepr db/J mice |
siTNF-α-loaded LNPs prepared as a potential wound treatment to combat an overzealous immune response and facilitate wound closure in a diabetic mouse model | • downregulated TNF-α gene expression • accelerated diabetic wound healing |
124 | |
| PHD2 | 8 mm full-thickness skin wound on the dorsal area of STZ-induced diabetic rats | • in vitro • in vivo SD rats |
PHD2 siRNA loaded in a porous poly(thioketal-urethane) scaffold | • downregulated PHD2 gene expression • accelerated diabetic wound healing • growth of thicker layer of skin tissue |
135 |
| GM3S | 6-mm full-thickness skin wound on the dorsal area of STZ-induced diabetic mice | • in vitro • in vivo male C57BL/6 mice |
GM3S siRNA loaded on gold NPs | • downregulated GM3S gene expression • accelerated diabetic wound healing • increased keratinocyte migration and proliferation |
136 |
| HIF-1 | • scratch test | • in vitro | HIF-1 siRNA loaded in layer-by-layer (LBL) self-assembled loaded gold NPs with poly L-arginine (AuNP@PLA) in the outer layer | • downregulated HIF-1 gene expression • upregulated pro-angiogenic pathways • accelerated wound healing in an in vitro scratch assay |
137 |
| IRF5 | • scratch test simulated inflammatory condition |
• in vitro | IRF5 siRNA loaded in selenium-based LBL NCs with polyethyleneimine (PEI) as the final polymer layer | • downregulated IRF5 gene expression. • decreased expression level of pro-inflammatory NOS-2 and TNF-α mRNA • increased expression level of the healing mediator Arg-1 • accelerated wound healing in an in vitro scratch assay |
138 |
siRNA, small interfering RNA; STZ, streptozotocin or streptozocin; MMP, matrix metalloproteinase; PEG, polyethylene glycol; Keap1, kelch-like ECH-associated protein 1; TNF-α, tumor necrosis factor alpha; MCP-1, monocyte chemoattractant protein 1; CCL2, chemokine (C-C motif) ligand 2; PHD2, prolyl hydroxylase domain protein 2; GM3S, ganglioside-monosialic acid 3 synthase; HIF-1, hypoxia-inducible factor 1; IRF5, interferon regulatory factor 5.
Recently, efforts to establish a more complete picture of the signaling pathways that define the final macrophage phenotype have uncovered the importance of the transcriptional regulator IRF5, which appears to have a central role in M1/M2 macrophage polarization.139 Abnormal levels of IRF5 have been linked to several inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease, and systemic lupus erythematosus.140, 141, 142 This is because IRF5 is a transcription factor that can bind to the regulatory regions of inflammatory genes to modulate their expression and, therefore, regulate macrophage polarization. Krausgruber et al.139 were the first to suggest that activation of IRF5 drives M1 macrophage polarization in such a way that IRF5 induces expression of 20 M1-specific genes and inhibits 19 M2-specific genes encoding cytokines, chemokines, costimulatory molecules, and surface receptors. This study indicated that IRF5 was indeed a major factor in defining macrophage polarization.139 These insights led to exploration of RNAi silencing of IRF5 in macrophages as a therapeutic strategy to promote resolution of inflammation and improving healing after myocardial infarction.143 siRNA targeting IRF5 (siIRF5) was formulated in LNPs and injected intravenously into atherosclerotic mice with myocardial infarction. The mice were injected daily for 4 days with 0.5 mg/kg siIRF5 or control siRNA (siCON). Treatment with siIRF5 resulted in efficient knockdown of IRF5 expression in macrophages and a subsequent decrease in expression of proinflammatory M1 markers, including TNF-α and IL-1β, without reducing M2 gene expression. Investigation of tissue biomarkers for infarct healing 7 days after myocardial infarction revealed a reduction in the number of neutrophils and macrophages, whereas neovascularization, the number of fibroblasts, and collagen deposition did not decline in mice treated with siIRF5.143 There are also reports demonstrating that increasing M2 macrophages through IRF siRNA treatment attenuates neural injury and neuropathic pain after spinal cord injury (SCI).144, 145, 146
The effect of IRF5 silencing on skin wound healing was first demonstrated in excisional skin wounds.143 Four days after creation of full-thickness skin wounds (6 mm in diameter), C57BL/6 mice intravenously received siIRF5 encapsulated in LNPs. The results indicated that injection of siIRF5 LNPs reduced IRF5 gene expression in macrophages isolated from the skin wounds in comparison with siCON. Wound closure was accelerated with decreased monocyte and neutrophil content accompanied by overall lower protease activity (Figures S3A–S3D). In addition, mRNA levels of TNF-α, IL-1β, and IL-6, of pro-inflammatory genes had decreased (Figure S3E). Another study found similar results in diabetic wounds, with a transition to the M2 phenotype after siIRF5 treatment. Thus, as expected, these changes in macrophage polarization were accompanied by increased expression of anti-inflammatory (CD206, CD163, IL-4, and IL-10) and reduced expression of inflammatory (CD11c, IL-1β, and TNF-α) markers.147
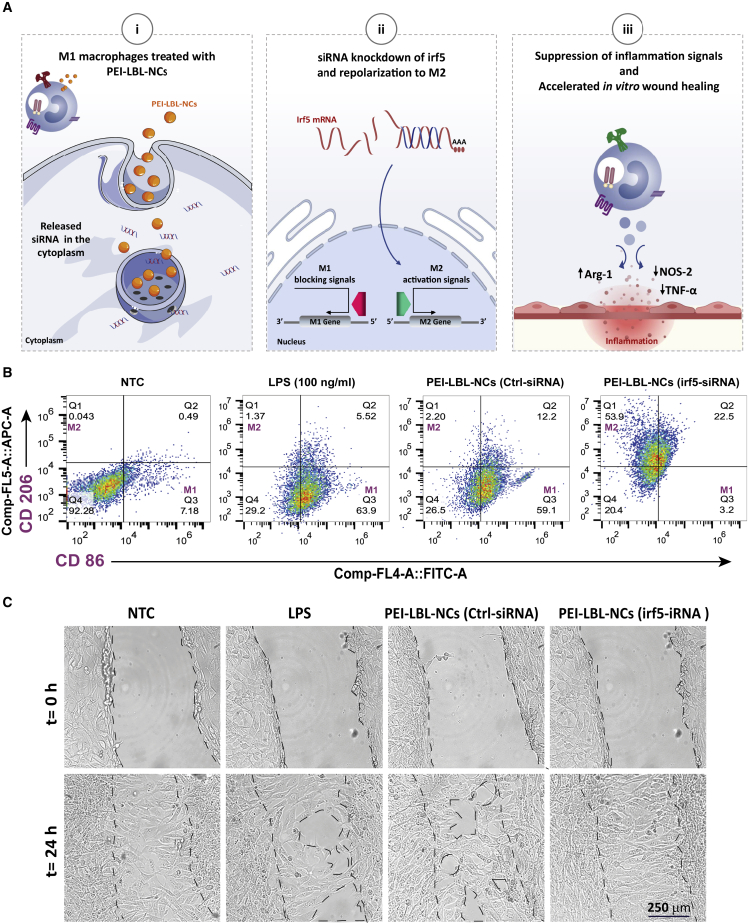
Recently, we explored M1-to-M2 macrophage repolarization using siIRF5 loaded in polyethyleneimine (PEI) layer-by-layer (LBL) nanocomplexes (NCs) (Figure 7A).138 Reprogramming of M1 macrophages to the M2 phenotype was confirmed by a shift in CD206 (M2) and CD86 (M1) expression with a decrease in the M1 macrophage population to 3%, whereas the percentage of M2 cells increased from 1% to 54% after treatment (Figure 7B), consistent with the work performed by other groups showing that IRF5 ablation inhibits M1 macrophage polarization and suppresses progression of inflammation.139,148,149 We demonstrated that the shift from the M1 to M2 phenotype accelerated wound healing in an in vitro scratch assay on fibroblast cells that were incubated with conditioned cell medium isolated from transfected macrophages (CD206+) (Figure 7C).138
Figure 7.
Enforced M1-to-M2 macrophage repolarization using siIRF5-loaded polyethyleneimine (PEI) layer-by-layer (LBL) nanocomplexes (NCs)
(A) i: M1 macrophage treated by IRF5 siRNA loaded in PEI LBL (NCs. ii: reprogramming into a pro-healing phenotype by successful release of IRF5 siRNA in the cytoplasm. iii: macrophage phenotype change from the M1 to the M2 phenotype leads to faster wound healing in an in vitro scratch assay. (B) Representative flow cytometry results showing the CD206+ (M2 marker) and CD86+ (M1 marker) populations in LPS-activated RAW 264.7 cells after treatment with PEI LBL NCs loaded with IFR5 siRNA or Ctrl (control) siRNA. (C) For the in vitro wound healing assay, the proliferation of NIH-3T3 fibroblasts was monitored by transmission microscopy after treatment with conditioned cell culture medium (C-CCM) containing secreted factors from macrophage cells (RAW 264.7) stimulated with LPS and treated with PEI LBL NCs loaded with IRF5-siRNA or Ctrl siRNA. Transmission microscopy images show NIH 3T3cells before (0 h) and 24 h after treatment with C-CCM from LPS-stimulated RAW 264.7 cells and LPS-stimulated RAW 264.7 cells treated with PEI LBL NCs loaded with IRF5 siRNA or Ctrl siRNA, NTC, non-treated control. Adapted from Sharifiaghdam et al.138
In a recent study,150 siIRF5 was encapsulated in fusogenic porous silicon NPs functionalized with the activated macrophage-targeting peptide CRV (F-siIRF5-CRV). It showed that effective depletion of the IRF5 gene in M1 macrophages (Figure S4A) could improve the therapeutic outcome of infected mice regardless of the bacterial strain and type. Mice with a methicillin-resistant Staphylococcus aureus (MRSA) muscle infection were used as a model for deep skin wound injuries. Although all treated groups had large abscess formations (purple) within the muscle tissue (pink), treatment with F-siIRF5-CRV resulted in only light and mild abscess formation on the right side of the tissue (Figure S4B). The majority of the section maintained a pink fibrous structure similar to that seen in the healthy control. The number of colony-forming units (CFUs) in the F-siIRF5-CRV treatment group was not statistically different from healthy controls (Figure S4C). The outcome of this study demonstrated that effective depletion of the IRF5 gene helped the immune system to focus on clearing of bacteria and minimizing auto-immune damage caused by excessive inflammation from M1 macrophages, improving the therapeutic outcome.150,151 The versatility of this approach was also demonstrated using mice with antibiotic-resistant Gram-positive (MRSA) and Gram-negative (Pseudomonas aeruginosa) skin/muscle infections, both of which are major contributors to the morbidity and mortality of hospital-acquired infection after wounding.152,153
Delivery of ex-vivo-activated macrophages
Ex-vivo-activated macrophages as a cell therapy for treatment of cancers has been investigated extensively since the 1980s.154 Isaiah Fidler is considered the founder of this approach because of his discovery of the anti-tumor effect of administration of ex-vivo-stimulated macrophages in a lung cancer mouse model,155,156 which he later expanded to non-cancerous applications.157 For example, one study that focused on local injection of polarized macrophages (M1) reported that, after administration, the cells promoted reperfusion recovery after femoral artery ligation in a mouse model because exogenous M1 macrophages decreased fibrosis and enhanced regeneration in lacerated muscles.158
It has been demonstrated in animal models that administration of ex-vivo-activated macrophages has anti-inflammatory functions and protect against Adriamycin-induced nephropathy in a murine model of chronic kidney injury.159 In the case of bone regeneration, it has also been shown that local injection of in-vitro-activated macrophages can improve vascularization and healing in bone-related injury160 and, in particular, that a combination of BM mononuclear cells (MNCs) and basic fibroblast growth factor (bFGF) gelatin hydrogel enhanced neovascularization and osteoinductive ability, resulting in bone regeneration. Delivery of activated M1 macrophages has also been reported for tissue healing applications. For example, it has been shown previously that administration of macrophages activated by hypo-osmotic shock promoted healing of pressure ulcers,154 and positive effects were also reported for accelerated vascularization, tissue repair, and cardiac remodeling early after myocardial infarction.161 The activated macrophages showed enhanced phagocytosis and augmented secretion of cytokines such as IL-1 and IL-6.162
A study has indicated that administration of autologous macrophages activated by TNF-α and IFN-γ (M1 phenotype) on day 1 after injury (non-chronic wound) can promote wound healing and normalize the defect in Vegf regulation associated with diabetes-induced skin repair disorders.163 On the other hand, administration of ex-vivo-activated murine macrophages to M2-like phenotypes with IL-4 or IL-10 during the early inflammatory phase after wounding resulted in impaired skin healing and delayed re-epithelialization in a diabetic mouse model, pointing out the importance of the delicate balance in macrophage phenotypes that should be present in the different phases of the wound healing process.164
There are clear indications that delivery of activated M1 macrophages during the early inflammation phase allows positive therapeutic outcomes for tissue regeneration under normal conditions (non-chronic wounds). However, topical administration of ex-vivo-activated macrophages (M1 or M2) for treatment of mice with long-term dermal wounds (chronic wounds) is associated with increased wound inflammation.165 This effect was tackled in a study that evaluated the influence of two murine macrophage subtypes on progression of chronic dermal wound healing. Macrophages were differentiated from embryonic stem cell-derived macrophages (ESDMs) and analyzed for their ability to function as non-inflammatory M2-like macrophages. ESDMs were compared with BM-derived macrophages (BMDM), which represented a more pro-inflammatory M1 macrophage subtype. The results of this study showed that ESDM- and BMDM-treated wounds displayed prolonged inflammation after administration instead of accelerating wound healing.165 These results are also in line with the delayed wound closure observed in the genetically diabetic mice, which was attributed to prolonged persistence of neutrophils and macrophages during the late phase of repair.166
Research and exploration with careful strategies that induce on-demand transitions of the local population of macrophage in the wound to generate the desired macrophage phenotype (less-inflammatory, pro-healing profile) could be more promising therapeutic approaches than administration of polarized macrophages, which can induce drastic perturbations in the wound microenvironment.
Conclusions and outlook
Macrophages play a decisive role in the skin wound healing process. They do so through their plasticity, which allows them to shift their phenotype and, thus, their role according to environmental cues during the various stages of the healing process. Dysregulation of macrophage function in any of the wound healing steps, such as a reduced ability to switch from the inflammation-related M1 to the pro-healing M2 phenotype, leads to a delay in wound closure. This can even lead to severe consequences; for instance, in the case of DFUs with increased risk of amputation. A gradual better understanding of the factors that regulate macrophage phenotypes has led to new therapeutic interventions attempting to normalize macrophage responses in chronic inflamed wounds. In this review, macrophage-based therapeutic strategies were divided into two main categories:
-
1
pharmacological approaches to influence macrophages directly at the wound site.
-
2
transplantation of ex-vivo-activated macrophages.
Regarding the latter, transplantation of ex-vivo-activated M1 macrophages directly into wounds has been highlighted for acceleration of wound healing under normal conditions, but in the context of diabetic wounds, administration of polarized macrophages can induce drastic perturbations in the wound microenvironment and delay wound closure. The functional stability of transferred macrophages in vivo it is not guaranteed, and it has been reported that their phenotype can change after administration.167 Therefore, although it is an intriguing new treatment modality, more in-depth studies of transferred macrophages over time are needed to better assess the feasibility of this approach.
Use of therapeutic molecules that can interfere with macrophage functionality directly at the wound site has certainly progressed. These molecules inhibit the activity of inflammatory M1-like macrophages and their effects or promote their transition to the M2 phenotype. Although administration of drug molecules leads to a reduction in inflammation and pro-healing wound conditions, their effect on the macrophage cell is not specific and could influence cells other than macrophages.168 Recent advances in the field of macrophage-based therapy at the wound site have shown that genetically manipulating macrophage phenotype by siRNA delivery allows precise control of key mediators inducing switches in the phenotype of macrophages in a highly sequence-specific manner. Genetic modification of macrophages is challenging, particularly under chronic wound conditions like diabetic wounds, but the advent of siRNA delivery by novel nanotechnology-based strategies provides a solution for these delivery obstacles. Despite the increasing volume of studies that support this strategy and the demonstrated improvements in chronic wounds, clinical translation of the research performed so far will require development of wound dressings that allow sustained release and delivery of siRNA that have so far been successful in guiding wound macrophages to the pre -healing phenotype, increasing healing mediators and growth factors in addition to preventing and treating any infection that might occur in the wound.
Acknowledgments
The authors would like to acknowledge funding from the European Union’s Horizon 2020 research and innovation program under grant agreements 810685 (DelNam project) and 648124 (NANOBUBBLE project). J.C.F. acknowledges the Flemish Research Foundation (FWO grants 1210120N and V407521N).
Author contributions
Conceptualization and writing – original draft, M.S.; writing – original draft, E.S.; supervision, R.F.-M. and S.D.S.; Writing – review & editing and supervision, K.B. and J.F.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.07.016.
Contributor Information
Kevin Braeckmans, Email: kevin.braeckmans@ugent.be.
Juan C. Fraire, Email: juan.fraire@ugent.be.
Supplemental information
References
- 1.Proksch E., Brandner J.M., Jensen J.M. The skin: an indispensable barrier. Exp. Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen A.V., Soulika A.M. The dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019;20:1811. doi: 10.3390/ijms20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodero M.P., Khosrotehrani K. Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 2010;3:643–653. [PMC free article] [PubMed] [Google Scholar]
- 4.Diegelmann R.F., Evans M.C. Wound healing: an overview of acute, fibrotic and delayed healing. Front. Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 5.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 6.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M., Hou Q., Zhong L., Zhao Y., Fu X. Macrophage related chronic inflammation in non-healing wounds. Front. Immunol. 2021;12:681710. doi: 10.3389/fimmu.2021.681710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark R.A. Fibrin is a many splendored thing. J. Invest. Dermatol. 2003;121 doi: 10.1046/j.1523-1747.2003.12575.x. xxi-xxii. [DOI] [PubMed] [Google Scholar]
- 9.Vannella K.M., Barron L., Borthwick L.A., Kindrachuk K.N., Narasimhan P.B., Hart K.M., Thompson R.W., White S., Cheever A.W., Ramalingam T.R., Wynn T.A. Incomplete deletion of IL-4Rα by LysMCre reveals distinct subsets of M2 macrophages controlling inflammation and fibrosis in chronic schistosomiasis. PLoS Pathog. 2014;10:e1004372. doi: 10.1371/journal.ppat.1004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raziyeva K., Kim Y., Zharkinbekov Z., Kassymbek K., Jimi S., Saparov A. Immunology of acute and chronic wound healing. Biomolecules. 2021;11:700. doi: 10.3390/biom11050700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuraitis D., Rosenthal N., Boh E., McBurney E. Macrophages in dermatology: pathogenic roles and targeted therapeutics. Arch. Dermatol. Res. 2022;314:133–140. doi: 10.1007/s00403-021-02207-0. [DOI] [PubMed] [Google Scholar]
- 12.Liu C., Wu C., Yang Q., Gao J., Li L., Yang D., Luo L. Macrophages mediate the repair of brain vascular rupture through direct physical adhesion and mechanical traction. Immunity. 2016;44:1162–1176. doi: 10.1016/j.immuni.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Novak M.L., Koh T.J. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 2013;183:1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shechter R., London A., Varol C., Raposo C., Cusimano M., Yovel G., Rolls A., Mack M., Pluchino S., Martino G., et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiPietro L.A. Angiogenesis and wound repair: when enough is enough. J. Leukoc. Biol. 2016;100:979–984. doi: 10.1189/jlb.4MR0316-102R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun B.K., Siprashvili Z., Khavari P.A. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–945. doi: 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- 17.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olczyk P., Mencner Ł., Komosinska-Vassev K. The role of the extracellular matrix components in cutaneous wound healing. Biomed. Res. Int. 2014;2014:747584. doi: 10.1155/2014/747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maquart F.X., Monboisse J.C. Extracellular matrix and wound healing. Pathol. Biol. 2014;62:91–95. doi: 10.1016/j.patbio.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Goren I., Allmann N., Yogev N., Schürmann C., Linke A., Holdener M., Waisman A., Pfeilschifter J., Frank S. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am. J. Pathol. 2009;175:132–147. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vickers N.J. Animal communication: when i’m calling you, will you answer too? Curr. Biol. 2017;27:R713–R715. doi: 10.1016/j.cub.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 22.Barman P.K., Urao N., Koh T.J. Diabetes induces myeloid bias in bone marrow progenitors associated with enhanced wound macrophage accumulation and impaired healing. J. Pathol. 2019;249:435–446. doi: 10.1002/path.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krzyszczyk P., Schloss R., Palmer A., Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018;9:419. doi: 10.3389/fphys.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder R.J., Driver V., Fife C.E., Lantis J., Peirce B., Serena T., Weir D. Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: a consensus panel discussion. Ostomy. Wound Manage. 2011;57:36–46. [PubMed] [Google Scholar]
- 25.Khanna S., Biswas S., Shang Y., Collard E., Azad A., Kauh C., Bhasker V., Gordillo G.M., Sen C.K., Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies L.C., Taylor P.R. Tissue-resident macrophages: then and now. Immunology. 2015;144:541–548. doi: 10.1111/imm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah J.M.Y., Omar E., Pai D.R., Sood S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012;45:220–228. doi: 10.4103/0970-0358.101282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz C., Gomez Perdiguero E., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K., Prinz M., Wu B., Jacobsen S.E.W., Pollard J.W., et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 29.Davies L.C., Jenkins S.J., Allen J.E., Taylor P.R. Tissue-resident macrophages. Nat. Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A., Biswas S.K., Galdiero M.R., Sica A., Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 31.Chitu V., Stanley E.R. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Hume D.A., MacDonald K.P.A. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–1820. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- 33.Minutti, C. M., Knipper, J. A., Allen, J. E. & Zaiss, D. M. (2016). Seminars in Cell & Developmental Biology. 3-11 (Elsevier). [DOI] [PubMed]
- 34.Wang Y., Szretter K.J., Vermi W., Gilfillan S., Rossini C., Cella M., Barrow A.D., Diamond M.S., Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graubardt N., Vugman M., Mouhadeb O., Caliari G., Pasmanik-Chor M., Reuveni D., Zigmond E., Brazowski E., David E., Chappell-Maor L., et al. Ly6Chi monocytes and their macrophage descendants regulate neutrophil function and clearance in acetaminophen-induced liver injury. Front. Immunol. 2017;8:626. doi: 10.3389/fimmu.2017.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Q., Choksi S., Qu J., Jang J., Choe M., Banfi B., Engelhardt J.F., Liu Z.G. NADPH oxidases are essential for macrophage differentiation. J. Biol. Chem. 2016;291:20030–20041. doi: 10.1074/jbc.M116.731216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crane M.J., Daley J.M., van Houtte O., Brancato S.K., Henry W.L., Jr., Albina J.E. The monocyte to macrophage transition in the murine sterile wound. PLoS One. 2014;9:e86660. doi: 10.1371/journal.pone.0086660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barman P.K., Pang J., Urao N., Koh T.J. Skin wounding–induced monocyte expansion in mice is not abrogated by IL-1 receptor 1 deficiency. J. Immunol. 2019;202:2720–2727. doi: 10.4049/jimmunol.1801481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu H., Xiong J., Qiu J., He X., Sheng H., Dai Q., Li D., Xin R., Jiang L., Li Q., et al. Type III secretion protein, PcrV, impairs Pseudomonas aeruginosa biofilm formation by increasing M1 macrophage-mediated anti-bacterial activities. Front. Microbiol. 2020;11:1971. doi: 10.3389/fmicb.2020.01971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallucci S. The Innate Immune Response to Noninfectious Stressors. Elsevier; 2016. An overview of the innate immune response to infectious and noninfectious stressors; pp. 1–24. [Google Scholar]
- 42.Yao Y., Xu X.-H., Jin L. Macrophage polarization in physiological and pathological pregnancy. Front. Immunol. 2019;10:792. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Won Y.-W., Patel A.N., Bull D.A. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. 2014;35:5627–5635. doi: 10.1016/j.biomaterials.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 44.Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front. Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang H.R., Milovanović M., Allan D., Niedbala W., Besnard A.G., Fukada S.Y., Alves-Filho J.C., Togbe D., Goodyear C.S., Linington C., et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages. Eur. J. Immunol. 2012;42:1804–1814. doi: 10.1002/eji.201141947. [DOI] [PubMed] [Google Scholar]
- 46.Daley J.M., Brancato S.K., Thomay A.A., Reichner J.S., Albina J.E. The phenotype of murine wound macrophages. J. Leukoc. Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livingston B., Crimi C., Newman M., Higashimoto Y., Appella E., Sidney J., Sette A. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J. Immunol. 2002;168:5499–5506. doi: 10.4049/jimmunol.168.11.5499. [DOI] [PubMed] [Google Scholar]
- 48.Peng H., Xian D., Liu J., Pan S., Tang R., Zhong J. Regulating the polarization of macrophages: a promising approach to vascular dermatosis. J. Immunol. Res. 2020;2020:8148272. doi: 10.1155/2020/8148272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gan J., Liu C., Li H., Wang S., Wang Z., Kang Z., Huang Z., Zhang J., Wang C., Lv D., Dong L. Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials. 2019;219:119340. doi: 10.1016/j.biomaterials.2019.119340. [DOI] [PubMed] [Google Scholar]
- 50.Fuchs E., Blau H.M. Tissue stem cells: architects of their niches. Cell Stem Cell. 2020;27:532–556. doi: 10.1016/j.stem.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porta, C., Riboldi, E., Ippolito, A. & Sica, A. (2015). Seminars in Immunology. 237-248 (Elsevier). [DOI] [PubMed]
- 52.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Ogle M.E., Segar C.E., Sridhar S., Botchwey E.A. Monocytes and macrophages in tissue repair: implications for immunoregenerative biomaterial design. Exp. Biol. Med. 2016;241:1084–1097. doi: 10.1177/1535370216650293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vannella K.M., Wynn T.A. Mechanisms of organ injury and repair by macrophages. Annu. Rev. Physiol. 2017;79:593–617. doi: 10.1146/annurev-physiol-022516-034356. [DOI] [PubMed] [Google Scholar]
- 55.Cui N., Hu M., Khalil R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delavary B.M., van der Veer W.M., van Egmond M., Niessen F.B., Beelen R.H. Niessen FB Beelen RHJ Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Potekaev N.N., Borzykh O.B., Medvedev G.V., Pushkin D.V., Petrova M.M., Petrov A.V., Dmitrenko D.V., Karpova E.I., Demina O.M., Shnayder N.A. The role of extracellular matrix in skin wound healing. J. Clin. Med. 2021;10:5947. doi: 10.3390/jcm10245947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nissinen L.M., Kähäri V.M. Collagen turnover in wound repair––A macrophage connection. J. Invest. Dermatol. 2015;135:2350–2352. doi: 10.1038/jid.2015.246. [DOI] [PubMed] [Google Scholar]
- 59.Xue M., Jackson C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care. 2015;4:119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aitcheson S.M., Frentiu F.D., Hurn S.E., Edwards K., Murray R.Z. Skin wound healing: normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules. 2021;26:4917. doi: 10.3390/molecules26164917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mirza R., DiPietro L.A., Koh T.J. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am. J. Pathol. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lucas T., Waisman A., Ranjan R., Roes J., Krieg T., Müller W., Roers A., Eming S.A. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 63.Mirza R., Koh T.J. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine. 2011;56:256–264. doi: 10.1016/j.cyto.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 64.Huang S.-M., Wu C.S., Chiu M.H., Wu C.H., Chang Y.T., Chen G.S., Lan C.C.E. High glucose environment induces M1 macrophage polarization that impairs keratinocyte migration via TNF-α: an important mechanism to delay the diabetic wound healing. J. Dermatol. Sci. 2019;96:159–167. doi: 10.1016/j.jdermsci.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Kimball A., Schaller M., Joshi A., Davis F.M., denDekker A., Boniakowski A., Bermick J., Obi A., Moore B., Henke P.K., et al. Ly6CHi blood monocyte/macrophage drive chronic inflammation and impair wound healing in diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2018;38:1102–1114. doi: 10.1161/ATVBAHA.118.310703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geiss L.S., Li Y., Hora I., Albright A., Rolka D., Gregg E.W. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult US population. Diabetes Care. 2019;42:50–54. doi: 10.2337/dc18-1380. [DOI] [PubMed] [Google Scholar]
- 67.Davidson M.B., Raskin P., Tanenberg R.J., Vlajnic A., Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr. Pract. 2011;17:395–403. doi: 10.4158/EP10323.OR. [DOI] [PubMed] [Google Scholar]
- 68.Bus S.A., Van Netten J.J., Hinchliffe R.J., Apelqvist J., Lipsky B.A., Schaper N.C., IWGDF Editorial Board Standards for the development and methodology of the 2019 international working group on the diabetic foot guidelines. Diabetes Metab. Res. Rev. 2020;36:e3267. doi: 10.1002/dmrr.3267. [DOI] [PubMed] [Google Scholar]
- 69.Ashcroft G.S., Jeong M.J., Ashworth J.J., Hardman M., Jin W., Moutsopoulos N., Wild T., McCartney-Francis N., Sim D., McGrady G., et al. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 2012;20:38–49. doi: 10.1111/j.1524-475X.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brüünsgaard H., Pedersen B.K. Age-related inflammatory cytokines and disease. Immunol. Allergy Clin. North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 71.Subramaniam K., Pech C.M., Stacey M.C., Wallace H.J. Induction of MMP-1, MMP-3 and TIMP-1 in normal dermal fibroblasts by chronic venous leg ulcer wound fluid. Int. Wound J. 2008;5:79–86. doi: 10.1111/j.1742-481X.2007.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Streit M., Beleznay Z., Braathen L.R. Topical application of the tumour necrosis factor-α antibody infliximab improves healing of chronic wounds. Int. Wound J. 2006;3:171–179. doi: 10.1111/j.1742-481X.2006.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Putte L., De Schrijver S., Moortgat P. The effects of advanced glycation end products (AGEs) on dermal wound healing and scar formation: a systematic review. Scars Burn. Heal. 2016;2 doi: 10.1177/2059513116676828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wicks, K., Torbica, T. & Mace, K. A. (2014). Seminars in Immunology. 341-353 (Elsevier). [DOI] [PubMed]
- 75.Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R.K., Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Q., Zhu G., Cao X., Dong J., Song F., Niu Y. Blocking AGE-RAGE signaling improved functional disorders of macrophages in diabetic wound. J. Diabetes Res. 2017:1428537. doi: 10.1155/2017/1428537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mirza R.E., Fang M.M., Ennis W.J., Koh T.J. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62:2579–2587. doi: 10.2337/db12-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodero M.P., Hodgson S.S., Hollier B., Combadiere C., Khosrotehrani K. Reduced Il17a expression distinguishes a Ly6cloMHCIIhi macrophage population promoting wound healing. J. Invest. Dermatol. 2013;133:783–792. doi: 10.1038/jid.2012.368. [DOI] [PubMed] [Google Scholar]
- 79.Yu Z.-W., Zhang J., Li X., Wang Y., Fu Y.H., Gao X.Y. A new research hot spot: the role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci. 2020;240:117138. doi: 10.1016/j.lfs.2019.117138. [DOI] [PubMed] [Google Scholar]
- 80.Liu D., Yang P., Gao M., Yu T., Shi Y., Zhang M., Yao M., Liu Y., Zhang X. NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin. Sci. 2019;133:565–582. doi: 10.1042/CS20180600. [DOI] [PubMed] [Google Scholar]
- 81.Lin H.-B., Wei G.S., Li F.X., Guo W.J., Hong P., Weng Y.Q., Zhang Q.Q., Xu S.Y., Liang W.B., You Z.J., Zhang H.F. Macrophage–NLRP3 inflammasome activation exacerbates cardiac dysfunction after ischemic stroke in a mouse model of diabetes. Neurosci. Bull. 2020;36:1035–1045. doi: 10.1007/s12264-020-00544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan Y., Das S., Li M. Vitamin D ameliorates impaired wound healing in streptozotocin-induced diabetic mice by suppressing NF-κB-mediated inflammatory genes. Biosci. Rep. 2018;38 doi: 10.1042/BSR20171294. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Yuan Y.F., Das S.K., Li M.Q. Vitamin D ameliorates impaired wound healing in streptozotocin-induced diabetic mice by suppressing endoplasmic reticulum stress. J. Diabetes Res. 2018:1757925. doi: 10.1155/2018/1757925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y., Wan L., Zhang Z., Li J., Qu M., Zhou Q. Topical calcitriol application promotes diabetic corneal wound healing and reinnervation through inhibiting NLRP3 inflammasome activation. Exp. Eye Res. 2021;209:108668. doi: 10.1016/j.exer.2021.108668. [DOI] [PubMed] [Google Scholar]
- 85.Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis. 2014;10:29–37. doi: 10.4161/org.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim J., Hematti P. Mesenchymal stem cell–educated macrophages: a novel type of alternatively activated macrophages. Exp. Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Németh K., Leelahavanichkul A., Yuen P.S.T., Mayer B., Parmelee A., Doi K., Robey P.G., Leelahavanichkul K., Koller B.H., Brown J.M., et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E 2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang S., Liu Y., Zhang X., Zhu D., Qi X., Cao X., Fang Y., Che Y., Han Z.C., He Z.X., et al. Prostaglandin E2 hydrogel improves cutaneous wound healing via M2 macrophages polarization. Theranostics. 2018;8:5348–5361. doi: 10.7150/thno.27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barminko J.A., Nativ N.I., Schloss R., Yarmush M.L. Fractional factorial design to investigate stromal cell regulation of macrophage plasticity. Biotechnol. Bioeng. 2014;111:2239–2251. doi: 10.1002/bit.25282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Q.Z., Su W.R., Shi S.H., Wilder-Smith P., Xiang A.P., Wong A., Nguyen A.L., Kwon C.W., Le A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sakai N., Van Sweringen H.L., Quillin R.C., Schuster R., Blanchard J., Burns J.M., Tevar A.D., Edwards M.J., Lentsch A.B. Interleukin-33 Is hepatoprotective during liver ischemia/reperfusion in mice. Hepatology. 2012;56:1468–1478. doi: 10.1002/hep.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miller A.M., Xu D., Asquith D.L., Denby L., Li Y., Sattar N., Baker A.H., McInnes I.B., Liew F.Y. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yin H., Li X., Hu S., Liu T., Yuan B., Gu H., Ni Q., Zhang X., Zheng F. IL-33 accelerates cutaneous wound healing involved in upregulation of alternatively activated macrophages. Mol. Immunol. 2013;56:347–353. doi: 10.1016/j.molimm.2013.05.225. [DOI] [PubMed] [Google Scholar]
- 94.Shao S., He F., Yang Y., Yuan G., Zhang M., Yu X. Th17 cells in type 1 diabetes. Cell. Immunol. 2012;280:16–21. doi: 10.1016/j.cellimm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Li S., Ding X., Zhang H., Ding Y., Tan Q. IL-25 improves diabetic wound healing through stimulating M2 macrophage polarization and fibroblast activation. Int. Immunopharmacol. 2022;106:108605. doi: 10.1016/j.intimp.2022.108605. [DOI] [PubMed] [Google Scholar]
- 96.Covarrubias A., Byles V., Horng T. ROS sets the stage for macrophage differentiation. Cell Res. 2013;23:984–985. doi: 10.1038/cr.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lurier E., Levy R., Barbee K., Golomb G., Spiller K.L. 41st Annual Northeast Biomedical Engineering Conference (NEBEC) IEEE. 2015;1-2 [Google Scholar]
- 98.Zhao H., Huang J., Li Y., Lv X., Zhou H., Wang H., Xu Y., Wang C., Wang J., Liu Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials. 2020;258:120286. doi: 10.1016/j.biomaterials.2020.120286. [DOI] [PubMed] [Google Scholar]
- 99.Escher P., Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat. Res. 2000;448:121–138. doi: 10.1016/s0027-5107(99)00231-6. [DOI] [PubMed] [Google Scholar]
- 100.Paakinaho V., Kaikkonen S., Levonen A.-L., Palvimo J.J. Electrophilic lipid mediator 15-deoxy-Δ12, 14-prostaglandin J2 modifies glucocorticoid signaling via receptor SUMOylation. Mol. Cell. Biol. 2014;34:3202–3213. doi: 10.1128/MCB.00748-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abdalla H.B., Napimoga M.H., Lopes A.H., de Macedo Maganin A.G., Cunha T.M., Van Dyke T.E., Clemente Napimoga J.T. Activation of PPAR-γ induces macrophage polarization and reduces neutrophil migration mediated by heme oxygenase 1. Int. Immunopharmacol. 2020;84:106565. doi: 10.1016/j.intimp.2020.106565. [DOI] [PubMed] [Google Scholar]
- 102.Jabbari P., Sadeghalvad M., Rezaei N. An inflammatory triangle in Sarcoidosis: PPAR-γ, Immune microenvironment, and Inflammation. Expert Opin. Biol. Ther. 2021;21:1451–1459. doi: 10.1080/14712598.2021.1913118. [DOI] [PubMed] [Google Scholar]
- 103.Bouhlel M.A., Derudas B., Rigamonti E., Dièvart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 104.Chen H., Shi R., Luo B., Yang X., Qiu L., Xiong J., Jiang M., Liu Y., Zhang Z., Wu Y. Macrophage peroxisome proliferator-activated receptor γ deficiency delays skin wound healing through impairing apoptotic cell clearance in mice. Cell Death Dis. 2015;6:e1597. doi: 10.1038/cddis.2014.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X., Sng M.K., Foo S., Chong H.C., Lee W.L., Tang M.B.Y., Ng K.W., Luo B., Choong C., Wong M.T.C., et al. Early controlled release of peroxisome proliferator-activated receptor β/δ agonist GW501516 improves diabetic wound healing through redox modulation of wound microenvironment. J. Control Release. 2015;197:138–147. doi: 10.1016/j.jconrel.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 106.Mirza R.E., Fang M.M., Novak M.L., Urao N., Sui A., Ennis W.J., Koh T.J. Macrophage PPARγ and impaired wound healing in type 2 diabetes. J. Pathol. 2015;236:433–444. doi: 10.1002/path.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Silva J.C., Pitta M.G.R., Pitta I.R., Koh T.J., Abdalla D.S.P. New peroxisome proliferator-activated receptor agonist (GQ-11) improves wound healing in diabetic mice. Adv. Wound Care. 2019;8:417–428. doi: 10.1089/wound.2018.0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerrick K.Y., Gerrick E.R., Gupta A., Wheelan S.J., Yegnasubramanian S., Jaffee E.M. Transcriptional profiling identifies novel regulators of macrophage polarization. PLoS One. 2018;13:e0208602. doi: 10.1371/journal.pone.0208602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vergadi E., Ieronymaki E., Lyroni K., Vaporidi K., Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017;198:1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 110.Fukuda D., Aikawa E., Swirski F.K., Novobrantseva T.I., Kotelianski V., Gorgun C.Z., Chudnovskiy A., Yamazaki H., Croce K., Weissleder R., et al. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc. Natl. Acad. Sci. USA. 2012;109:E1868–E1877. doi: 10.1073/pnas.1116889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Radtke F., MacDonald H.R., Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat. Rev. Immunol. 2013;13:427–437. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 112.Nakano T., Fukuda D., Koga J.-i., Aikawa M. Delta-like ligand 4-notch signaling in macrophage activation. Arterioscler. Thromb. Vasc. Biol. 2016;36:2038–2047. doi: 10.1161/ATVBAHA.116.306926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amici S.A., Dong J., Guerau-de-Arellano M. Molecular mechanisms modulating the phenotype of macrophages and microglia. Front. Immunol. 2017;8:1520. doi: 10.3389/fimmu.2017.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ning S., Pagano J.S., Barber G.N. IRF7: activation, regulation, modification and function. Genes Immun. 2011;12:399–414. doi: 10.1038/gene.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ikushima H., Negishi H., Taniguchi T. 105-116. Cold Spring Harbor Laboratory Press; 2013. (Cold Spring Harbor Symposia on Quantitative Biology). [DOI] [PubMed] [Google Scholar]
- 116.Günthner R., Anders H.-J. Interferon-regulatory factors determine macrophage phenotype polarization. Mediators Inflamm. 2013;2013:731023. doi: 10.1155/2013/731023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carthew R.W., Sontheimer E.J. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gomes M.J., Martins S., Sarmento B. siRNA as a tool to improve the treatment of brain diseases: mechanism, targets and delivery. Ageing Res. Rev. 2015;21:43–54. doi: 10.1016/j.arr.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 119.Huang C., Li M., Chen C., Yao Q. Small interfering RNA therapy in cancer: mechanism, potential targets, and clinical applications. Expert Opin. Ther. Targets. 2008;12:637–645. doi: 10.1517/14728222.12.5.637. [DOI] [PubMed] [Google Scholar]
- 120.Guo P., Coban O., Snead N.M., Trebley J., Hoeprich S., Guo S., Shu Y. Engineering RNA for targeted siRNA delivery and medical application. Adv. Drug Deliv. Rev. 2010;62:650–666. doi: 10.1016/j.addr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gao H., Cheng R., A Santos H. Nanoparticle-mediated siRNA delivery systems for cancer therapy. View. 2021;2:20200111. [Google Scholar]
- 122.Kasiewicz L.N., Whitehead K.A. Silencing TNFα with lipidoid nanoparticles downregulates both TNFα and MCP-1 in an in vitro co-culture model of diabetic foot ulcers. Acta Biomater. 2016;32:120–128. doi: 10.1016/j.actbio.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 123.Zhang J., Yang X., Wang H., Zhao B., Wu X., Su L., Xie S., Wang Y., Li J., Liu J., et al. PKCζ as a promising therapeutic target for TNFα-induced inflammatory disorders in chronic cutaneous wounds. Int. J. Mol. Med. 2017;40:1335–1346. doi: 10.3892/ijmm.2017.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kasiewicz L.N., Whitehead K.A. Lipid nanoparticles silence tumor necrosis factor α to improve wound healing in diabetic mice. Bioeng. Transl. Med. 2019;4:75–82. doi: 10.1002/btm2.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang J., Park Y., Zhang H., Gao X., Wilson E., Zimmer W., Abbott L., Zhang C. Role of MCP-1 in tumor necrosis factor-α-induced endothelial dysfunction in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1208–H1216. doi: 10.1152/ajpheart.00396.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li N., Luo H.C., Yang C., Deng J.J., Ren M., Xie X.Y., Lin D.Z., Yan L., Zhang L.M. Cationic star-shaped polymer as an siRNA carrier for reducing MMP-9 expression in skin fibroblast cells and promoting wound healing in diabetic rats. Int. J. Nanomed. 2014;9:3377–3387. doi: 10.2147/IJN.S66368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lan B., Zhang L., Yang L., Wu J., Li N., Pan C., Wang X., Zeng L., Yan L., Yang C., Ren M. Sustained delivery of MMP-9 siRNA via thermosensitive hydrogel accelerates diabetic wound healing. J. Nanobiotechnol. 2021;19:130. doi: 10.1186/s12951-021-00869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li N., Luo H.C., Ren M., Zhang L.M., Wang W., Pan C.L., Yang L.Q., Lao G.J., Deng J.J., Mai K.J., et al. Efficiency and safety of β-CD-(D3) 7 as siRNA carrier for decreasing matrix metalloproteinase-9 expression and improving wound healing in diabetic rats. ACS Appl. Mater. Inter. 2017;9:17417–17426. doi: 10.1021/acsami.7b02809. [DOI] [PubMed] [Google Scholar]
- 129.Du Y., Yang X., Gong Q., Xu Z., Cheng Y., Su G. Inhibitor of growth 4 affects hypoxia-induced migration and angiogenesis regulation in retinal pigment epithelial cells. J. Cell. Physiol. 2019;234:15243–15256. doi: 10.1002/jcp.28170. [DOI] [PubMed] [Google Scholar]
- 130.Li N., Yang L., Pan C., Saw P.E., Ren M., Lan B., Wu J., Wang X., Zeng T., Zhou L., et al. Naturally-occurring bacterial cellulose-hyperbranched cationic polysaccharide derivative/MMP-9 siRNA composite dressing for wound healing enhancement in diabetic rats. Acta Biomater. 2020;102:298–314. doi: 10.1016/j.actbio.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 131.Lan B., Wu J., Li N., Pan C., Yan L., Yang C., Zhang L., Yang L., Ren M. Hyperbranched cationic polysaccharide derivatives for efficient siRNA delivery and diabetic wound healing enhancement. Int. J. Biol. Macromol. 2020;154:855–865. doi: 10.1016/j.ijbiomac.2020.03.164. [DOI] [PubMed] [Google Scholar]
- 132.Yan L.-P., Castaño I.M., Sridharan R., Kelly D., Lemoine M., Cavanagh B.L., Dunne N.J., McCarthy H.O., O'Brien F.J. Collagen/GAG scaffolds activated by RALA-siMMP-9 complexes with potential for improved diabetic foot ulcer healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;114:111022. doi: 10.1016/j.msec.2020.111022. [DOI] [PubMed] [Google Scholar]
- 133.Kim H.S., Son Y.J., Yoo H.S. Clustering siRNA conjugates for MMP-responsive therapeutics in chronic wounds of diabetic animals. Nanoscale. 2016;8:13236–13244. doi: 10.1039/c6nr01551d. [DOI] [PubMed] [Google Scholar]
- 134.Rabbani P.S., Zhou A., Borab Z.M., Frezzo J.A., Srivastava N., More H.T., Rifkin W.J., David J.A., Berens S.J., Chen R., et al. Novel lipoproteoplex delivers Keap1 siRNA based gene therapy to accelerate diabetic wound healing. Biomaterials. 2017;132:1–15. doi: 10.1016/j.biomaterials.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 135.Martin J.R., Nelson C.E., Gupta M.K., Yu F., Sarett S.M., Hocking K.M., Pollins A.C., Nanney L.B., Davidson J.M., Guelcher S.A., Duvall C.L. Local delivery of PHD2 siRNA from ROS-degradable scaffolds to promote diabetic wound healing. Adv. Healthc. Mater. 2016;5:2751–2757. doi: 10.1002/adhm.201600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Randeria P.S., Seeger M.A., Wang X.Q., Wilson H., Shipp D., Mirkin C.A., Paller A.S. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc. Natl. Acad. Sci. USA. 2015;112:5573–5578. doi: 10.1073/pnas.1505951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shaabani E., Sharifiaghdam M., Lammens J., De Keersmaecker H., Vervaet C., De Beer T., Motevaseli E., Ghahremani M.H., Mansouri P., De Smedt S., et al. Increasing angiogenesis factors in hypoxic diabetic wound conditions by siRNA delivery: additive effect of LbL-gold nanocarriers and desloratadine-induced lysosomal escape. Int. J. Mol. Sci. 2021;22:9216. doi: 10.3390/ijms22179216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sharifiaghdam M., Shaabani E., Sharifiaghdam Z., De Keersmaecker H., Lucas B., Lammens J., Ghanbari H., Teimoori-Toolabi L., Vervaet C., De Beer T., et al. Macrophage reprogramming into a pro-healing phenotype by siRNA delivered with LBL assembled nanocomplexes for wound healing applications. Nanoscale. 2021;13:15445–15463. doi: 10.1039/d1nr03830c. [DOI] [PubMed] [Google Scholar]
- 139.Krausgruber T., Blazek K., Smallie T., Alzabin S., Lockstone H., Sahgal N., Hussell T., Feldmann M., Udalova I.A. IRF5 promotes inflammatory macrophage polarization and TH 1-TH 17 responses. Nat. Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 140.Weiss M., Blazek K., Byrne A.J., Perocheau D.P., Udalova I.A. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediators Inflamm. 2013;2013:245804. doi: 10.1155/2013/245804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang Y., Zhang C., Jing D., He H., Li X., Wang Y., Qin Y., Xiao X., Xiong H., Zhou G. IRF5 acts as a potential therapeutic marker in inflammatory bowel diseases. Inflamm. Bowel Dis. 2021;27:407–417. doi: 10.1093/ibd/izaa200. [DOI] [PubMed] [Google Scholar]
- 142.Almuttaqi H., Udalova I.A. Advances and challenges in targeting IRF5, a key regulator of inflammation. FEBS J. 2019;286:1624–1637. doi: 10.1111/febs.14654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Courties G., Heidt T., Sebas M., Iwamoto Y., Jeon D., Truelove J., Tricot B., Wojtkiewicz G., Dutta P., Sager H.B., et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J. Am. Coll. Cardiol. 2014;63:1556–1566. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li J., Liu Y., Xu H., Fu Q. Nanoparticle-delivered IRF5 siRNA facilitates M1 to M2 transition, reduces demyelination and neurofilament loss, and promotes functional recovery after spinal cord injury in mice. Inflammation. 2016;39:1704–1717. doi: 10.1007/s10753-016-0405-4. [DOI] [PubMed] [Google Scholar]
- 145.Terashima T., Ogawa N., Nakae Y., Sato T., Katagi M., Okano J., Maegawa H., Kojima H. Gene therapy for neuropathic pain through siRNA-IRF5 gene delivery with homing peptides to microglia. Mol. Ther. Nucleic Acids. 2018;11:203–215. doi: 10.1016/j.omtn.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alizadeh A., Dyck S.M., Karimi-Abdolrezaee S. Myelin damage and repair in pathologic CNS: challenges and prospects. Front. Mol. Neurosci. 2015;8:35. doi: 10.3389/fnmol.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Al-Rashed F., Sindhu S., Arefanian H., Al Madhoun A., Kochumon S., Thomas R., Al-Kandari S., Alghaith A., Jacob T., Al-Mulla F., Ahmad R. Repetitive intermittent hyperglycemia drives the M1 polarization and inflammatory responses in THP-1 macrophages through the mechanism involving the TLR4-IRF5 pathway. Cells. 2020;9:1892. doi: 10.3390/cells9081892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dalmas E., Toubal A., Alzaid F., Blazek K., Eames H.L., Lebozec K., Pini M., Hainault I., Montastier E., Denis R.G.P., et al. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat. Med. 2015;21:610–618. doi: 10.1038/nm.3829. [DOI] [PubMed] [Google Scholar]
- 149.Seneviratne A.N., Edsfeldt A., Cole J.E., Kassiteridi C., Swart M., Park I., Green P., Khoyratty T., Saliba D., Goddard M.E., et al. Interferon regulatory factor 5 controls necrotic core formation in atherosclerotic lesions by impairing efferocytosis. Circulation. 2017;136:1140–1154. doi: 10.1161/CIRCULATIONAHA.117.027844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim B., Yang Q., Chan L.W., Bhatia S.N., Ruoslahti E., Sailor M.J. Fusogenic porous silicon nanoparticles as a broad-spectrum immunotherapy against bacterial infections. Nanoscale Horiz. 2021;6:330–340. doi: 10.1039/d0nh00624f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim B., Pang H.B., Kang J., Park J.H., Ruoslahti E., Sailor M.J. Immunogene therapy with fusogenic nanoparticles modulates macrophage response to Staphylococcus aureus. Nat. Commun. 2018;9:1969. doi: 10.1038/s41467-018-04390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.DeLeon S., Clinton A., Fowler H., Everett J., Horswill A.R., Rumbaugh K.P. Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model. Infect. Immun. 2014;82:4718–4728. doi: 10.1128/IAI.02198-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sydnor E.R.M., Perl T.M. Hospital epidemiology and infection control in acute-care settings. Clin. Microbiol. Rev. 2011;24:141–173. doi: 10.1128/CMR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zuloff-Shani A., Adunsky A., Even-Zahav A., Semo H., Orenstein A., Tamir J., Regev E., Shinar E., Danon D. Hard to heal pressure ulcers (stage III–IV): efficacy of injected activated macrophage suspension (AMS) as compared with standard of care (SOC) treatment controlled trial. Arch. Gerontol. Geriatr. 2010;51:268–272. doi: 10.1016/j.archger.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 155.Fidler I.J. Inhibition of pulmonary metastasis by intravenous injection of specifically activated macrophages. Cancer Res. 1974;34:1074–1078. [PubMed] [Google Scholar]
- 156.West M.A., Bennet T., Seatter S.C., Clair L., Bellingham J. LPS pretreatment reprograms macrophage LPS-stimulated TNF and IL-1 release without protein tyrosine kinase activation. J. Leukoc. Biol. 1997;61:88–95. doi: 10.1002/jlb.61.1.88. [DOI] [PubMed] [Google Scholar]
- 157.Wang Y., Wang Y.P., Zheng G., Lee V.W.S., Ouyang L., Chang D.H.H., Mahajan D., Coombs J., Wang Y.M., Alexander S.I., Harris D.C.H. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–299. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 158.Jetten N., Donners M.M.P.C., Wagenaar A., Cleutjens J.P.M., van Rooijen N., de Winther M.P.J., Post M.J. Local delivery of polarized macrophages improves reperfusion recovery in a mouse hind limb ischemia model. PLoS One. 2013;8:e68811. doi: 10.1371/journal.pone.0068811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lu J., Cao Q., Zheng D., Sun Y., Wang C., Yu X., Wang Y., Lee V.W.S., Zheng G., Tan T.K., et al. Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int. 2013;84:745–755. doi: 10.1038/ki.2013.135. [DOI] [PubMed] [Google Scholar]
- 160.Hisatome T., Yasunaga Y., Yanada S., Tabata Y., Ikada Y., Ochi M. Neovascularization and bone regeneration by implantation of autologous bone marrow mononuclear cells. Biomaterials. 2005;26:4550–4556. doi: 10.1016/j.biomaterials.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 161.Leor J., Rozen L., Zuloff-Shani A., Feinberg M.S., Amsalem Y., Barbash I.M., Kachel E., Holbova R., Mardor Y., Daniels D., et al. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation. 2006;114:I94–I100. doi: 10.1161/CIRCULATIONAHA.105.000331. [DOI] [PubMed] [Google Scholar]
- 162.Frenkel O., Shani E., Ben-Bassat I., Brok-Simoni F., Rozenfeld-Granot G., Kajakaro G., Rechavi G., Amariglio N., Shinar E., Danon D. Activated macrophages for treating skin ulceration: gene expression in human monocytes after hypo-osmotic shock. Clin. Exp. Immunol. 2002;128:59–66. doi: 10.1046/j.1365-2249.2002.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gu X.-y., Shen S.e., Huang C.f., Liu Y.n., Chen Y.c., Luo L., Zeng Y., Wang A.p. Effect of activated autologous monocytes/macrophages on wound healing in a rodent model of experimental diabetes. Diabetes Res. Clin. Pract. 2013;102:53–59. doi: 10.1016/j.diabres.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 164.Jetten N., Roumans N., Gijbels M.J., Romano A., Post M.J., de Winther M.P.J., van der Hulst R.R.W.J., Xanthoulea S. Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses. PloS one. 2014;9:e102994. doi: 10.1371/journal.pone.0102994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dreymueller D., Denecke B., Ludwig A., Jahnen-Dechent W. Embryonic stem cell–derived M2-like macrophages delay cutaneous wound healing. Wound Repair Regen. 2013;21:44–54. doi: 10.1111/j.1524-475X.2012.00858.x. [DOI] [PubMed] [Google Scholar]
- 166.Wetzler C., Kämpfer H., Stallmeyer B., Pfeilschifter J., Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J. Invest. Dermatol. 2000;115:245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 167.Lavin Y., Winter D., Blecher-Gonen R., David E., Keren-Shaul H., Merad M., Jung S., Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chamberlain C.S., Leiferman E.M., Frisch K.E., Wang S., Yang X., Brickson S.L., Vanderby R. The influence of interleukin-4 on ligament healing. Wound Repair Regen. 2011;19:426–435. doi: 10.1111/j.1524-475X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.