Figure 2.
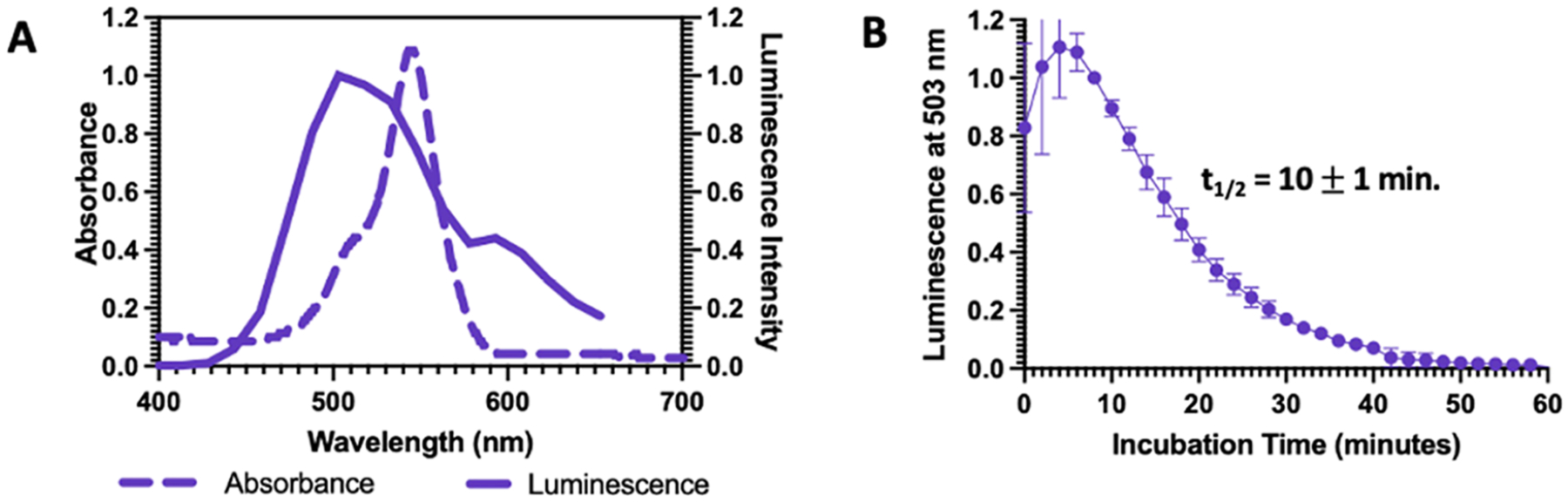
Evidence of CRET demonstrated by luminescence and absorbance spectral comparisons and half-lives. (A) Normalized absorption spectrum of CL-E1 (dashed line) overlayed with its normalized chemiluminescence spectrum (solid line) in PBS pH 7.4 (5% DMSO) showing that chemiluminescence resonance energy transfer is possible. (B) Chemiluminescence time course of CL-E1 (20 μM) plotted at its luminescence wavelength maximum and normalized to 1 at the time of maximum luminescence intensity. Half-life of dioxetane breakdown was measured to be 10 ± 1 min in PBS pH 7.4 (5% DMSO). Dioxetane half-life in PBS pH 7.4 (5% DMSO) for CL-E1 is shorter compared to control probes CL-A and CL-PN (Figure S4), suggesting that energy transfer occurs instead of energy release via luminescence. Measurements were performed in triplicate using independent samples.
