FIGURE 2.
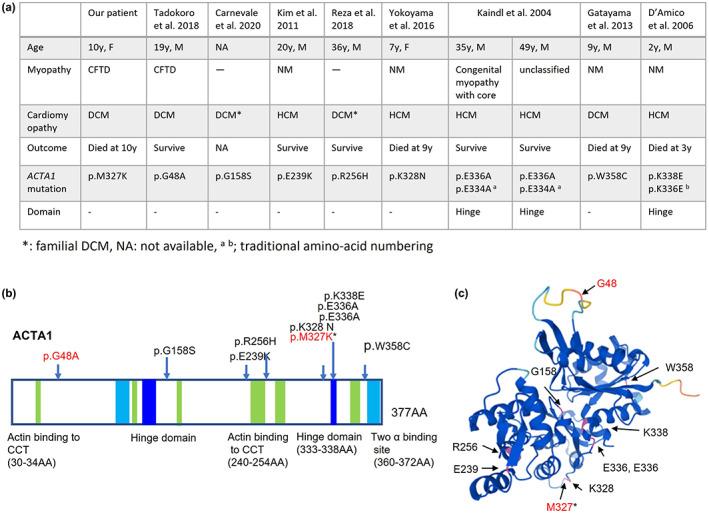
Clinical features of cardiomyopathy with ACTA1 mutations (a), schematic representation of the ACTA1 gene (b), and three‐dimensional (3D) model of the ACTA1 monomer (c). (b)The hinge domains are at 137–150 amino acids (AA) and 333–338 AA. Two actin‐binding sites are at 112–125 AA and 360–372 AA. Sites of actin‐binding to cytosolic chaperonin containing TCP‐1 (CCT) are at 30–34 AA, 135–139 AA, 170–174 AA, 240–254 AA, 265–274 AA, and 340–349 AA. (b, c) Arrows indicate the mutations in Figure 2a. The mutations written in red are associated with congenital fiber‐type disproportion (CFTD). Asterisk indicates our patient. The ACTA1 monomer has been illustrated by AlphaFold Protein Structure Database (https://alphafold.ebi.ac.uk/).
