Abstract
Previous studies have shown mixed results on the relationship between prenatal, birth, and postnatal (“pregnancy-related”) risk factors and attention-deficit/hyperactivity disorder (ADHD). We conducted meta-analyses to identify potentially modifiable pregnancy-related factors associated with ADHD. A comprehensive search of PubMed, Web of Science, and EMBASE in 2014, followed by an updated search in January 2021, identified 69 articles published in English on pregnancy-related risk factors and ADHD for inclusion. Risk factors were included in the meta-analysis if at least three effect sizes with clear pregnancy-related risk factor exposure were identified. Pooled effect sizes were calculated for ADHD overall, ADHD diagnosis, inattention, and hyperactivity/impulsivity. Odds ratios (OR) were calculated for dichotomous measures and correlation coefficients (CC) for continuous measures. Prenatal factors (pre-pregnancy weight, preeclampsia, pregnancy complications, elevated testosterone exposure), and postnatal factors (Apgar score, neonatal illness, no breastfeeding) were positively associated with ADHD overall; the findings for ADHD diagnosis were similar with the exception that there were too few effect sizes available to examine pre-pregnancy weight and lack of breastfeeding. Prenatal testosterone was significantly associated with inattention and hyperactivity/impulsivity. Effect sizes were generally small (range 1.1–1.6 ORs, −0.16–0.11 CCs). Risk factors occurring at the time of birth (perinatal asphyxia, labor complications, mode of delivery) were not significantly associated with ADHD. A better understanding of factors that are consistently associated with ADHD may inform future prevention strategies. The findings reported here suggest that prenatal and postnatal factors may serve as potential targets for preventing or mitigating the symptoms of ADHD.
Attention-deficit/hyperactivity disorder (ADHD) in childhood is characterized by developmentally inappropriate levels of inattention, hyperactivity, and impulsivity that significantly interfere with learning and social relationships. As outlined in the 5th edition of the Diagnostic and Statistical Manual for Mental Disorders (DSM-5; American Psychiatric Association, 2013), symptoms must present by the age of 12 years, interfere with functioning in two or more settings, and not be better explained by another mental disorder such as anxiety disorder or schizophrenia. Although the specific diagnostic criteria have evolved over time (e.g., DSM-IV criteria required that symptoms present before the age of seven years rather than 12 years), the core features of inattention, hyperactivity and impulsivity have been retained. ADHD has been diagnosed in 9.4% of children aged 2–17 years in the United States (Danielson et al., 2018) and has significant public health relevance based on its impact throughout the lifespan on overall health, social relationships, education, and employment (Erskine et al., 2016; Schoenfelder & Kollins, 2016). Efforts to reduce the impact of ADHD could thus benefit individuals, families, schools, communities, and society.
Although ADHD is known to have a significant genetic contribution, many environmental and experiential risk factors have been shown to be associated with increased risk for ADHD (Milberger et al., 1997; Froehlich et al., 2011; Faraone et al., 2021). Findings on the association of specific risk factors with ADHD are often mixed, possibly due to different methodologies across studies. For example, many studies rely on cross-sectional designs, leaving questions about whether the ADHD symptoms or the risk factor exposure occurred first. Another source of heterogeneity across studies is that published manuscripts frequently rely on different measures of ADHD (e.g., symptom counts versus diagnostic cutoffs) or how the risk factor of interest was measured (e.g., parent report versus medical records). A more comprehensive understanding of potentially modifiable risk factors for ADHD may lead to prevention efforts that target reductions in the impact of inattention, hyperactivity, and impulsivity at both the individual and public health levels.
Maternal health before and during pregnancy plays a critical role in the development of healthy newborns and can have lasting effects on children’s physical and mental development (Dean & Davis, 2007; Marques et al., 2015; Sullivan et al., 2014). Pregnancy-related factors including prenatal, birth, and postnatal factors have been reported in individual studies to be linked to numerous negative outcomes, including neurodevelopmental disorders and behavioral problems (Dean & Davis, 2007; Sullivan et al., 2014). Numerous mechanisms, including hypoxia (Smith et al., 2016), inflammation (Instanes et al., 2017), nutrition (Sullivan et al., 2014), and exposure to atypical hormone levels (e.g., elevated testosterone, Martel et al., 2008; Roberts & Martel, 2013; Silva et al., 2014), have been proposed as the pregnancy-related factors linked with brain development which could be related to the expression of ADHD symptoms. In addition, because pregnancy-related factors may be associated with maternal mental and overall health, the relationship between these factors and the onset of ADHD symptoms in childhood may also be influenced by shared genetics.
Research on the relationship between pregnancy-related factors and ADHD has taken place for over 40 years, with mixed findings on the association between specific risk factors and symptoms of ADHD. Pregnancy-related risk factors affecting child development with potential to increase risk for ADHD in children include prenatal factors (e.g., pregnancy complications), events occurring at birth (e.g., labor complications) and postnatal factors (e.g., no breastfeeding). Although a large literature exists on preterm birth and low birth weight as risk factors for neurodevelopmental disorders, including ADHD (see Sciberras et al., 2017), risk factors with potential for more targeted prevention strategies were prioritized for these analyses. Furthermore, specific risk factors for preterm birth (Goldenberg et al., 2008), including maternal weight, pre-eclampsia, maternal stress (Robinson et al., this issue), and prenatal drug exposure (Maher et al., under review in this issue), were included in the current series (this article and others in the same special issue) of meta-analyses. A better understanding of risk factors that are consistently associated with ADHD may inform future prevention strategies. In this study, we use meta-analysis to summarize the findings across studies of pregnancy-related factors and indicators of ADHD, including ADHD diagnosis, inattention, and hyperactivity/impulsivity.
Methods
Document Search, Retrieval, and Coding
This manuscript serves as the first in a six-part series that presents findings from meta-analyses conducted on the association between six risk factor categories and ADHD. This manuscript reports on pregnancy-related risk factors, and subsequent manuscripts in the series report on the following risk factor categories: chemical (Dimitrov et al., under review in this issue), parent mental health (Robinson et al., this issue), parent substance use disorders (Maher et al., under review in this issue), parenting (Claussen et al., this issue), and child health factors (So et al., under review in this issue). All manuscripts in the series are based on identical core methods; only this manuscript will describe the series’ methodologic components in detail. Explanations of additional methods that are specific to this manuscript are labeled here as pregnancy-related.
In January–February, 2014, we searched PubMed, Web of Science (WOS), and EMBASE for studies reporting on associations between potentially modifiable risk factors and ADHD diagnosis or symptoms. This review was not registered and a review protocol was not prepared. We employed two types of search strategies, a targeted risk factor search and a general ADHD risk study approach. The targeted risk factor search was designed to identify studies of ADHD and specific potential risk factors known to the authors from the literature or linked with ADHD in popular media. Search strings included variants of ADHD terms (e.g., attention deficit, ADHD, hyperactivity) combined with specific suspected risk factor terms (e.g., preterm birth, pesticide, trauma). To capture risk factors potentially missed by the targeted approach, we also employed a more general search to identify any additional relevant studies of risk for ADHD. Search strings included the same variants of ADHD terms used in the targeted searches (e.g., attention deficit, ADHD, hyperactive) combined with methods and analytic terms that identify studies of risk (e.g., exposure, odds). All searches were restricted to terms that appeared in the titles or abstracts, to human research participants, and publications in English. No restriction was placed on publication date. The full set of search strings for the second strategy is provided in a Supplementary table. Relevant publications discovered through iterative reference mining of retrieved articles were subsequently added to the collection of eligible studies. Specific search terms for pregnancy-related risk factors used in the initial search included weight, obesity, BMI, body mass index, blood pressure, labetalol, toxemia, eclampsia, preeclampsia, long duration of labor, fetal distress, Apgar, Pitocin, Syntocinon, oxytocin, birth (AND (date OR month OR season)), indue*, labor, low birth weight, low birthweight, LBW, premature, preterm, intrauterine growth, birth AND complicate, hemorrhage. Articles on breastfeeding and prenatal exposure to testosterone were identified from the results of the general search strategy and from iterative reference mining.
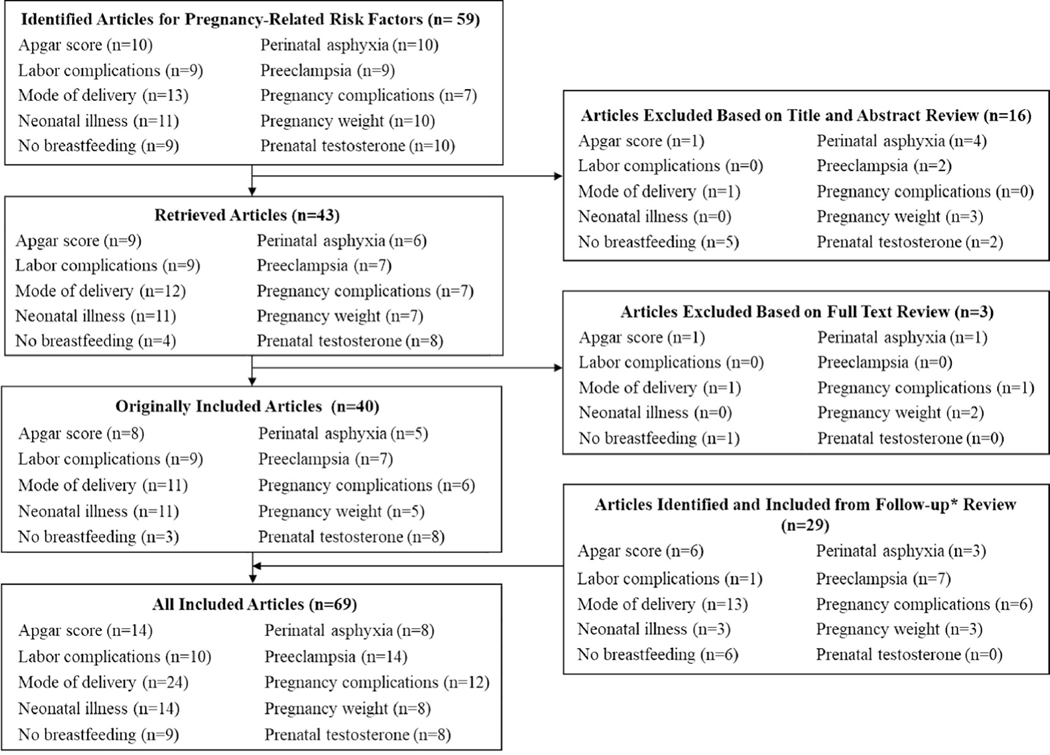
No automation tools were used to identify articles for inclusion or exclusion. Publications identified in the searches were reviewed for inclusion or exclusion in three stages (see Fig. 1 for pregnancy-related study triage). Studies were excluded at the first stage if the title or abstract clearly identified the article as out of scope, such as studies of memory loss related to aging, evaluations of interventions or treatments, and studies of ADHD as a risk factor for later outcomes. All articles included after title and abstract review, including articles about which coders were unsure, were retrieved for full-text review. Additional reasons for exclusion during full-text review included investigations of risk factors outside the scope of this meta-analysis (e.g., genetic studies) and non-empirical publications (i.e., theoretical or review articles). Data from all publications included after the second stage were extracted for potential analysis. Studies were excluded if they used the same population to examine the same risk factor; in these cases, the earlier publication was included in the analysis unless a later publication reported on a larger sample of the same population. Publications were also excluded during or after data extraction as necessary, for example, if sufficient data with which to compute a standardized effect size were not reported.
Fig-1.
Flowchart of triage process for articles identified for meta-analyses of prenatal, birth, and postnatal risk factors associated with attention-deficit/hyperactivity disorder
Note: Articles overlap across categories and therefore the n representing the sum of the individual risk factors is greater than the overall n for each box. Articles were identified through a comprehensive search of PubMed, Web of Science, and EMBASE and iterative reference mining in 2014, and an updated search of PubMed, Web of Science, and EMBASE in January 2021.
Because the risk factors of interest in this meta-analysis were ethically inappropriate for experimental manipulation, all eligible studies were non-experimental and thus studies of association rather than causation. To rule out the potential that ADHD caused or influenced the purported risk factor, we included only longitudinal or retrospective studies in which the measurement time period for the risk factor clearly preceded the measurement time period for ADHD. Among pregnancy-related risk factors, the notable exception was prenatal testosterone, which was measured by finger-length ratio in childhood. Studies using this methodology were included because it is widely accepted that finger-length ratio is a strong indicator of prenatal testosterone exposure levels (Lutchmaya et al., 2004).
Coders were trained and tested to criterion before data extraction began. During the first phase, the double-coding rate for articles was set to be at least 25%. After coders demonstrated consistent success using the coding forms, the minimum double-coding rate was reduced to 15%. Inter-rater agreement was continuously monitored via inter-rater reliability metrics and Cohen’s kappa, and coders were retrained if the coefficient was less than 0.70. A series of hierarchical coding forms (available from the authors) were used to capture all relevant data from each publication. A study-level form included questions concerning basic publication identification information, inclusion criteria verification, key study methods and setting information, and study population characteristics. An outcome-level form captured characteristics of the ADHD outcome, including how it was measured, the case definition (if applicable), and at what age the measurement occurred. Multiple outcome-level forms were used when more than one ADHD measure was included in a study. Effect-size-level forms captured characteristics of the risk factor exposure and the statistical relationship reported between the risk factor and ADHD outcome. Effect size information was extracted whenever possible, regardless of whether the risk factor was the main variable of interest in a study or was simply included as a covariate or statistical control.
Meta-Analysis Methods
All meta-analyses were conducted using R versions 3.1.3 through 4.0.3 (R Core Team, 2015–2020) using the rmeta package (Lumley, 2018). We calculated standardized summary statistics and variances for each relevant result within each included study using conventional meta-analytic techniques. For continuous outcomes, results including group means, sample sizes, and standard deviations were transformed into a t-statistic, such that:
where x represents the exposed or unexposed group mean, s2 represents a group standard deviation and n a group sample size. Results reported as a non-standardized regression coefficient (β) allowed for a t-statistic calculated as:
where se represents the standard error. Results were also included if a standardized regression coefficient (β) or correlation coefficient was reported. All t-statistics were converted to a correlation coefficient (r) for meta-analysis as follows:
For dichotomous outcomes, odds ratios were calculated. When effect sizes with standard errors, confidence intervals, or sample sizes were available, but not raw count data, the study was included and represented by its effect size and standard error.
When multiple effect sizes for the same risk factor and type of ADHD outcome were reported in a study (e.g., if data from two different measures of hyperactivity were reported, or if results were reported separately for boys and girls), the average of the relevant effect sizes was used, reflecting the relationship between a risk factor and type of ADHD outcome. When multiple effect sizes were reported for different time points (i.e., risk factor or ADHD outcome measured at different ages), only one time point was selected for analysis; in these cases, the ADHD outcomes closer in time to the risk factor measurement (but at least 6 months apart, to maintain the integrity of the longitudinal measurement inclusion criteria) were selected, to maximize the analyses’ ability to detect an effect if present. When selecting among multiple time points for risk factor measurements, we similarly selected the later age measurement (but at least 6 months prior to the ADHD measurement). The exception to that rule was when multiple prenatal time periods were reported for a risk factor, in which case we selected the earliest measurement, because earlier exposures are generally associated with the greatest impact on neurodevelopment (Rice & Barone, 2000). When a single article reported analyses from different samples (e.g., three distinct longitudinal studies), effect sizes were treated as separate studies for these meta-analyses.
For continuous or dichotomous outcomes, pooled effect sizes and 95% confidence intervals were calculated via a complete pooling approach. Each effect size was weighted by its conditional variance (Hedges & Olkin, 1985) to give more weight to studies with larger sample sizes. The variance across effect sizes was assessed by calculating a heterogeneity statistic, Q, which describes the variation across study estimates (DerSimonian & Laird, 1986). We fit separate random-effects models for each set of ADHD risk factors. Random-effects models include a weighting term (tau) to account for the between-study variation in effect size (Sutton et al., 2000), thus the random-effects model is considered to produce a more conservative estimate of effect size (i.e., pulled toward the null) than a fixed-effect model (Berlin et al., 1989).
Results are presented for each analysis for which there were at least three measures of association (hereafter referred to as effect sizes) between a particular risk factor and ADHD. Our meta-analyses were conducted separately for statistics with outcomes that were measured dichotomously (e.g., ADHD diagnosis) versus outcomes that were measured continuously (e.g., ADHD symptoms). Some risk factors only had a sufficient number (i.e.., at least three) of independent effect sizes to produce an overall effect size for either dichotomous or continuous statistics. Whenever possible (i.e., when at least three relevant effect sizes were available), additional analyses of subsets of studies within a risk factor category were conducted. For a pregnancy-related example, 12 studies reporting dichotomous statistics were included for pregnancy complications, 10 of which relied on ADHD diagnosis as the outcome measure. We were thus able to compute effect sizes between pregnancy complications and 1) any ADHD measure (referred to as “overall”) and 2) specific to ADHD diagnosis (referred to as “diagnosis only”). Similarly, for the general risk factor category “mode of delivery,” we had 24 articles, six of which were specific to breech delivery. Thus, we were able to compute test statistics for the more general mode of delivery and the more specific breech delivery risk factor. Among the pregnancy-related factors, only prenatal testosterone exposure had at least three effect sizes for separate analyses of the inattentive and hyperactive-impulsive symptoms that characterize ADHD.
In January, 2021, we conducted a literature search using the same search criteria as the original review, to identify papers published from 2014 through early 2021. We restricted our inclusion criteria to categories of risk factors where we had already generated effect sizes, based on the methodology described above.
Results
A total of 59 articles related to prenatal, birth, and postnatal risk factors were identified through the original directed searches and iterative reference mining. Most of the articles were identified through the initial directed searches for each risk factor except for testosterone, for which six of 10 articles reviewed were identified through iterative reference mining. After excluding 16 articles based on title and abstract review and three additional articles based on full-text review, a total of 40 articles published between 1979 and 2014 were included. An additional 29 articles were included based on the second literature search conducted in 2021, for a total of 69 articles included (Fig. 1). Three articles included two separate study samples; therefore, data are presented on 72 samples. Table 1 includes information about each article including sample size, basic demographics of the study sample (i.e., age, sex, country), and how ADHD and perinatal risk factors were measured. Many articles included findings for multiple categories of risk factors (e.g., pregnancy complications and neonatal illness; see Table 1).
Table 1.
Characteristics of studies included in meta-analyses of prenatal, birth, and postnatal risk factors for attention-deficit/hyperactivity disorder
| Study | Risk Factors (Included) | Sample size | Age at Outcome Measurement (Years) | Male (%) | ADHD Measurement (Included) | Sample (country) | Measurement |
|---|---|---|---|---|---|---|---|
| Ben Amor et al. (2005) | Labor Complications; Neonatal Illness | 100 | 8.8–10.1 | 34–90 | Diagnosis (clinical, DSM-IV) | Clinical, outpatient research survey/interview including siblings without ADHD as control (Canada) | Retrospective parent report of perinatal factors |
| Bhatia et al. (1991) | Mode of Delivery; Pregnancy Complications | 224 | 3–12 | 85.7 | Diagnosis (clinical, DSM-III) | Clinical, outpatient research survey/interview of children screened in pediatric hospital (India) | Retrospective parent report of pregnancy-related factors |
| Böhm et al. (2019) | Preeclampsia | 13,192 | 7 | 50.6 | Parent report of physician-diagnosed ADHD; SDQP | Cohort Study (MCS) (United Kingdom) | Maternal report |
| Bos-Veneman et al. (2010) | Labor Complications; Neonatal Illness | 65 | 12.2 | 88 | ADHD symptoms (ADHD Rating Scale-TV)P | Clinical, outpatient research survey/interview of children identified in psychiatric clinic (The NetherlandsNetherlandsNetherlands) | Retrospective parent report of perinatal factors |
| Brionet al. (2011) | Pregnancy Weight | 4,873 | 3–4 (47 months) | n/a | ADHD symptoms (SDQ)P | Birth cohort (ALSPAC; United Kingdom) | Self-report of pre-pregnancy weight at enrollment during pregnancy |
| Pregnancy Weight | 2,483 | 3 | n/a | Inattention (CBCL attention problems)P | Birth cohort (Generation R; The Netherlands) | Self-report of pre-pregnancy weight at enrollment postpartum | |
| Chandola et al. (1992) | Apgar Score; Neonatal Illness; Pregnancy Complications | 24,672–24,785 | 3–6 | 51–68.2 | Hyperactivity (Referral to clinic for hyperactivity) | Birth cohort (CBS; Wales) | Pregnancy-related information recorded on birth records prior to maternity hospital discharge |
| Chen et al. (2019) | Perinatal Asphyxia | 4,750 | 10.8 | 77.5 | Clinical diagnosis (ICD-9) | Taiwan Longitudinal Health Insurance Database (Taiwan) | Health insurance database |
| Claycomb et al. (2004) | Labor Complications; Mode of Delivery | 130 | 8.49–10.65 | n/a | Diagnosis (ADHD prescription medication bottle) | Research survey with recruitment through advertisements (USA) | Retrospective parent report of perinatal factors |
| Clements et al. (2015) | Apgar Score, Mode of Delivery | 7,874 | 2–19 | 71.2–73.5 | Diagnosis (ICD-9, DSM-IV) | Matched case control study using electronic health records (USA) | Electronic health records |
| Curran et al. (2016) | Mode of Delivery | 1,722,548 | <3 | 51.4 | Diagnosis (ICD-10) or medication treatment | Cohort study using linked data from National Patient Register and Multi-generation Register (Sweden) | Medical birth register |
| Dachew et al. (2019) | Preeclampsia | 6,597 | 7 | n/a | Diagnosis (DAWBA, DSM-IV)P | ALSPAC (United Kingdom) | Medical record review |
| de Bruin et al. (2006) | Testosterone | 153 | 9.08 | 100 | Diagnosis (clinical, DSM-IV) | Clinical, outpatient research study of children identified in psychiatric clinic; controls recruited from a school (The Netherlands) | Right hand 2nd:4th digit ratio |
| D’Souza et al. (2019) | No Breastfeeding | 6,246 | 2 | n/a | Inattention/hyperactivity (SDQ)P | Longitudinal prospective birth cohort (Growing Up in New Zealand; New Zealand) | Prospective maternal report |
| Fianu and Joelsson (1979) | Mode of Delivery | 1,921 | n/a | n/a | ADHD symptoms (Impulsivity, Hyperkinetic syndrome. Retrospective report of behavior from birth to study date)P | Case reports of premature and breech delivery collected at birth (Sweden) | Retrospective parent report of behavior and achievement from birth to study date |
| Fink et al. (2007) | Testosterone | 58 | 5–7 | 43.1 | Hyperactivity (SDQ)P | School based research study (United Kingdom) | Right hand 2nd:4th digit ratio |
| Testosterone | 56 | 6–11 | 51.8 | Inattention (CBCL attention problems)P | School based research study (Austria) | Right hand 2nd:4th digit ratio | |
| Froehlich et al. (2009) | Neonatal Illness | 2,528 | 8–15 | 63.5 | Diagnosis (DISC-IV, or parent report of previous diagnosis, and treatment with ADHD medication in past year)P | National survey (NHANES; USA) | Retrospective parent report of care in NICU |
| Getahun et al. (2013) | Apgar Score; Labor Complications; Mode of Delivery; Neonatal Illness; Perinatal Asphyxia; Preeclampsia; Pregnancy Complications |
81,678 | 8 | 50.5–74.3 | Diagnosis (medical record of diagnosis and at least 2 ADHD prescriptions) | Nested case-control study (USA) | Medical records (Kaiser Permanente Southern California) |
| Guhn et al. (2020) | Apgar Score, Mode of Delivery | 134,094 | 5.7 | 51 | ADHD symptoms (EDI)T | Cohort study using linked records (Canada) | Linked population-based vital statistics (birth records), and child survey data |
| Gurevitz (2014) | Mode of Delivery | 116 | 7.77–8.17 | 65.5–69 | Diagnosis (clinical, DSM-IV) | Retrospective study (Israel) | Review of well-baby clinic records |
| Gustafsson and Källen (2011) | Apgar Score; Mode of Delivery; Preeclampsia | 32,012 | 5–17 | 51–87 | Diagnosis (clinical, DSM-m-R or DSM-IV) | Case-control study linking medical records (Sweden) | Medical birth registry |
| Gustavson et al. (2019) | Pregnancy Complications |
99,947 | 7.4–17.3 | 51.2 | ADHD diagnosis (ICD-10); ADHD rating scale (DSM-IV) | Norwegian Mother and Child Cohort Study (Norway) | Prospective maternal report |
| Han et al. (2015) | Labor Complications | 19,940 | Elementary school age (<9—>12) | 49.4 | ADHD symptoms (Korean version of the DuPaul Rating Scale, K-ARS)P | School-based survey of parents (South Korea) | Parent report |
| Hanć et al. (2018) | Apgar Score | 278 | 6–18 | 100 | Diagnosis (ICD-10, DSM-IV; Conners’ Parent Rating Scale, Diagnostic Structured Interview for ADHD and Hyperkinetic Disorder) | Poland | Medical registry |
| Hoffman et al. (2010) | Neonatal Illness | 578 | 12–15 | 47.7–80.4 | Diagnosis (parent report of previous diagnosis)P | National survey (NHANES; USA) | Retrospective parent report of care in NICU |
| Ji et al. (2018) | Mode of Delivery, Pregnancy Complications | 1,479 | 7–12 | 48.1 | Diagnosis (ICD-9, ICD-10 codes in medical records) | Prospective birth cohort study using medical records (BBC; USA) | Medical records |
| Jin et al. (2014) | Mode of Delivery, Perinatal Asphyxia | 5,648 | 5–15 | 39.2 | Clinical ADHD diagnosis (DSM-IV) | School-based sample, Zhabei District, Shanghai (China) | Retrospective parent report |
| Jo et al. (2015) | Pregnancy Weight | 1,311 | 6 | 49.9 | Diagnosis (Parent report) and ADHD symptoms (SDQ)P | Nationally distributed longitudinal study (IFPS II, USA) | Maternal report |
| Joelsson et al. (2016) | Apgar Score | 48,943 | 6–20 | n/a | ADHD diagnosis (ICD-9, ICD-10) | Nationwide register study based on a nested case-control design (Finland) | Linkages of three national registers: FHDR FMBR, Finnish Central Population Register |
| Julvez et al. (2007) | No Breastfeeding | 199 | 4 | 50.2 | ADHD symptoms (ADHD-DSM-IV questionnaire)T | Birth cohort (Spain) | Maternal report of breastfeeding obtained by questionnaire at 1 and 4 years, or at 6 months, 14 months, and 2 years (child age) |
| Kadziela-Olech and Piotrowska-Jastrzebska (2005) | Apgar Score; Labor Complications; Pregnancy Complications | 100 | 7.3–7.8 | 85 | Diagnosis (Behavioral Scale of ICD-10 criteria)P, T | School-based Research study (Poland) | Retrospective parent report of pregnancy-related factors |
| Küllén et al. (2011) | Apgar Score; Pregnancy Weight; Mode of Delivery; Preeclampsia | 78,250–681,292 | n/a | n/a | Diagnosis (prescription of methylphenidate or atomoxetine recorded in drug register) | Data linkage with medical records (Sweden) | Medical birth registry |
| Kimetal. (2009) | No Breastfeeding; Mode of Delivery; Neonatal Illness | 2,419 | n/a | 48.6 | Diagnosis (DISC-IV)P | School-based research study (Seoul Child and Adolescent Mental Health Survey; Korea) | Retrospective parent report of pregnancy-related factors |
| Kosidou et al. (2017) | Apgar Score; Preeclampsia; Pregnancy Weight | 558,910 | 3–17 | 68.8 | ADHD diagnosis or treatment (ICD-10) | Matched case-control study using health and population data registers for all children bom in Sweden from 1984 to 2008 (Sweden) | Medical Birth Register (MBR) |
| Li et al. (2011) | Apgar Score | 7,246,218 | ≥3 | 82 | Diagnosis (ICD-10 diagnosis of hyperkinetic disorder recorded in one of two national registers) | Population-based cohort study (Sweden) | Medical birth registry |
| Lin et al. (2017) | Perinatal Asphyxia, Mode of Delivery | 21,243 | 1.3–5.7 | 54.6 | Hyperactivity (CPRS-48)P | School-based cohort Study (LCCS; China) | Questionnaire |
| Linnet et al. (2006) | Apgar Score | 1,355 | 3.5 | n/a | Hyperactivity (PBQ)P | Birth cohort (Denmark) | Midwife report at delivery |
| Liu et al. (2012) | Testosterone | 219 | 11 | 54.8 | Inattention (CBCL; TRF)P, T | School-based research study (China) | Right hand 2nd:4th digit ratio |
| Maher et al. (2020) | Preeclampsia | 10,692 | 3 | 51.4 | ADHD symptoms (SDQ)P | Nationally representative longitudinal study of children (GUI; Ireland) | Questionnaire, interview (maternal-report) |
| Mann and McDermott (2011) | Preeclampsia | 84,721 | 6.49 | 46.7–71.9 | Diagnosis (ICD-9, Medicaid billing data) | Data linkage with medical records (USA) | Medicaid billing data |
| Martel et al. (2008) | Testosterone | 250 | 14.67 | 56.2–63.7 | Diagnosis (clinical, K-SADS-E) and symptoms (K-SADS-E; ADHD Rating Scale)P,T | Clinical research study (USA) | Right hand 2nd:4th digit ratio |
| Martel (2009) | Testosterone | 312 | 13.31 | 50–63.1 | Diagnosis (clinical, K-SADS-E) and ADHD symptoms (ADHD Rating Scales; BASC,CRS)P,T | School, clinic, and community based research study (USA) | Right hand 2nd:4th digit ratio |
| McFadden et al. (2005) | Testosterone | 31–61 | 7–15 | 60.7 | Diagnosis (inattentive or combined type, DSM-IV-TR) | Clinical research study (USA) | Right hand 2nd:4th digit ratio |
| McIntosh et al. (1995) | Labor Complications | 209 | 9.5–10.4 | 53–85 | Diagnosis (DSM-III-R) | School-based research study (USA) | Retrospective parent report of perinatal factors (The Maternal Perinatal Scale) |
| Melchior et al. (2015) | No Breastfeeding | 1,113 | 5 | 51.4–53.4 | ADHD symptoms (SDQ)P | DEN mother-child cohort study (France) | Maternal report questionnaire |
| Mimouni-Bloch et al. (2013) | No Breastfeeding | 159 | 9.5–10.36 | 50.5–73.2 | Diagnosis (clinical) | Clinic-based research study, with sibling and non-psychiatric clinical control groups (Israel) | Retrospective parent report of breastfeeding |
| Motlagh et al. (2010) | Pregnancy Weight; Perinatal Asphyxia; Preeclampsia; Pregnancy Complications | 117 | 11.8–12.2 | 46–73 | Diagnosis (clinical, SADS-PLV) | Clinic-based research study, with population-based controls (USA) | Retrospective parent report of pregnancy and perinatal factors |
| Murray et al. (2016) | Apgar Score, Mode of Delivery | 6,849 | 7 | n/a | ADHD symptoms (SDQ), Diagnosis (DAWBA)P | ALSPAC Birth cohort (United Kingdom) | Birth records |
| Apgar Score, Mode of Delivery | 3,509 | 7 | n/a | ADHD symptoms (SDQ), Diagnosis (DAWBA)P | Pelotas birth cohort (Brazil) | Maternal interview | |
| Park et al. (2014a) | Neonatal Illness | 900 | 6–15 | 73.8–85.4 | ADHD diagnosis (DSM-IV; K-SADS-PL, DISC-IV)P | Research study with clinical recruitment of ADHD sample from hospital and school-based non-ADHD sample (South Korea) | Parent report |
| Park et al. (2014b) | No Breastfeeding | 874 | 8–11 | 58.2 | ADHD Diagnosis (DSM-IV; DISC-IV)P | School sample across five regions (South Korea) | Maternal report |
| Pineda et al. (2007) | Neonatal Illness; Perinatal Asphyxia; Preeclampsia; Pregnancy Complications | 486 | 7.9–8.3 | 47.2–74.5 | Diagnosis (DSM-IV; DICA-PR, BASC, DSM-IV ADHD Symptom Questionnaire, ADHD Checklist; all were being treated with methylphenidate) | Clinic-based research study; controls recruited from schools (Colombia) | Retrospective parent report of pregnancy-related factors as part of BASC |
| Pires et al. (2013) | Neonatal Illness | 366 | 7.9 | 50.8 | ADHD diagnosis/symptoms (CBCL, TRF)P, T | School-based research study (Brazil) | Retrospective maternal report of neonatal illness |
| Pohlabelnetal. (2017) | Mode of Delivery, Preeclampsia | 15,577 | 2–12 | 50.6 | Parent report of diagnosis | European IDEFICS prospective multicenter cohort study (Belgium, Cyprus, Estonia, Germany, Hungary, Italy, Spain and Sweden) | Parent questionnaire |
| Pringsheim et al. (2009) | Mode of Delivery; Perinatal Asphyxia | 353 | 9.9–10 | 76.7–83.4 | Diagnosis (clinical, DSM-IV-TR) | Clinic-based case-control research study. Cases had ADHD+Tourette syndrome (TS); controls had TS only (Canada) | Retrospective report of perinatal factors included as part of demographic form for all new patients |
| Roberts & Martel, 2013 | Testosterone | 109 | 4.82 | 46.7–72.2 | ADHD symptoms (K-DBDS, DSM-IV)P | Community-based research study (USA) | Right hand 2nd:4th digit ratio |
| Rodriguez et al. (2008) | Pregnancy Weight | 12,976 | 7–12 | 50 | ADHD symptoms (SDQ; Rutter)T | Follow-up of pregnancy cohorts (Sweden, Denmark, Finland) | Pre-pregnancy weight collected prospectively |
| Rodriguez (2010) | Pregnancy Weight | 907 | 5 | 53 | ADHD symptoms (DSM-IV, SDQ)P, T | Follow-up of pregnancy cohort (Sweden) | Early pregnancy weight recorded in Swedish Medical Birth Register |
| Russell et al. (2014) | Mode of Delivery | 8,443 | 7.2 | 50.3–82.2 | Diagnosis (parent report of previous diagnosis)P | Birth cohort (MCS; United Kingdom) | UK Birth Registration and Maternity Hospital Episode Data |
| Say et al. (2016) | Mode of Delivery, Preeclampsia, Pregnancy Complications | 180 | 3–18 | 71–78 | Clinical diagnosis (DSM-IV) | Case-control study recruited from pediatric clinics and child/adolescent psychiatry clinics (Turkey) | Maternal report |
| Sciberras et al. (2011) | Neonatal Illness | 3,477 | 6.8 | 51 | Diagnosis (parent report of previous diagnosis)P | Nationally representative population-based birth cohort (LSAC; Australia) | Retrospective parent report of whether the child required intensive care at birth |
| Shih et al. (2020) | Mode of Delivery | 16,376 | 8 | 52.5 | Diagnosis (parent report of previous diagnosis)P | National prospective longitudinal cohort study (Taiwan) | Parent report |
| Silva et al. (2014) | Apgar Score; Labor Complications; Mode of Delivery; Perinatal Asphyxia; Preeclamp- sia |
43,062 | 0–25 | 77 | Diagnosis (use of stimulant medication recorded in MODDS) | Population-based, record linkage case-control study (Australia) | Prospectively ascertained pregnancy and perinatal information obtained from MNS |
| Stadler et al. (2016) | No Breastfeeding | 474 | 7–13 | 63.9 | Diagnosis (DSM-5; KSAD-S-E, Conners, DuPaul ADHD Rating Scale IV)P | Case control cohort (USA) | Maternal report |
| St. Sauver et al. (2004) | Labor Complications | 5,631 | n/a | 50.1–75.4 | Diagnosis (algorithm based on ADHD symptoms, diagnosis, use of stimulants) | Birth cohort (USA) | Birth certificate (perinatal information) records linked to school and medical records |
| Stevenson et al. (2007) | Testosterone | 187 | n/a | 27.8 | ADHD symptoms (DSM-IV)S | School (college)-based research study (USA) | Right hand 2nd:4th digit ratio |
| Sucksdorff et al. (2018) | Preeclampsia, Pregnancy Complications, Mode of Delivery, Neonatal Illness | 49,533 | 6–20 | n/a | ADHD diagnosis (ICD-9, ICD-10) | Nested case-control study based on the Finnish Prenatal study of ADHD (Finland) | Linkages of three national registers: FHDR FMBR, Finnish Central Population Register |
| Wagner et al. (2009) | Labor Complications; Neonatal Illness | 748 | 8.13 | 49.2 | ADHD symptoms (HBQ, CBQ, DISC-IV)P | Wisconsin Twin Panel (USA) | Perinatal risk factors coded from medical records using OCS, NCS |
| Wang et al. (2019) | Mode of Delivery, No Breastfeeding | 401 | 8.6 | 79–84 | Diagnosis, (SNAP and clinical interview; DSM-IV)P | Community based case-control study (China) | Face to face interview |
| Wiggs et al. (2016) | Pregnancy Complications, Neonatal Illness | 464 | 6–17 | 55 | Diagnosis (DSM-IV ADHD Rating Scale, Conners’ Rating Scale - Revised Short Form, K-SADS-E)P,T,S | Research study recruited from community and clinics (USA) | Parent report |
| Zhu et al. (2015) | Mode of Delivery, No Breastfeeding, Pregnancy Complications, Pregnancy Weight |
1,765 | 4 | 54 | ADHD symptoms (Conners’ Hyperactivity IndexP | Research study recruited prenatally from hospital (China) | Parent interview and medical records |
ADHDT The Attention Deficit Hyperactivity Disorder Test, ALSPAC Avon Longitudinal Study of Parents and Children, BASC Behavior Assessment System for Children, BBC Boston Birth Cohort, C-ASQ Conners abbreviated symptom questionnaire, CBCL Child Behavior Checklist, CBQ Children Behavior Questionnaire, CBS Cardiff Birth Survey, CPRS-48 Conners’ Parent Rating Scale-Revised, CRS Conners Rating Scale, DAWBA Development and Wellbeing Assessment, DEN Étude sur les Déterminants pré - et postnatals pré coces du développement psychomoteur et de la santé de l’Enfant, DICA-PR Diagnostic Interview for Children and Adolescents - Parent Revised Version, DSM-IIIR Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, DSM-TV Diagnostic and Statistical Manual of Mental Disorders, 4th edition, DSM-TV-TR Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision, DSM-5 Diagnostic and Statistical Manual of Mental Disorders, 5th edition, EDI Early Development Instrument, FHDR Finnish Hospital Discharge Register, FMBR Finnish Medical Birth Register, GUI Growing Up in Ireland, HBQ MacArthur Health and Behavior Questionnaire, IFPSII The Infant Feeding Practices Study n, IDEFICS study Identification and prevention of dietary- and lifestyle-induced health effects in children and infants, K-DBDS Kiddie-Disruptive Behavior Disorder Schedule, K-SADS-E Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children, Epidemiological Version, LCCS The Longhua Child Cohort Study, LSAC Longitudinal Study of Australian Children, MCS Millennium Cohort Study, MNS Midwives Notification System, MODDS Monitoring of Drugs of Dependence System, n/a not available, NCS Neonatal Complications Scale, NHANES National Health and Nutrition Examination Survey, NICU Neonatal intensive care unit, OCC Odense Child Cohort, OCS Obstetrical Complications Scale, PBQ Preschool Behavior Questionnaire, SADS-PLV Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version, SDQ Strengths and Difficulties Questionnaire, TBCS Taiwan Birth Cohort Study, TRF Teacher Report Form
Parent report;
Teacher report;
Self report
The perinatal risk factors included in this study, and the number of eligible articles for each were: pre-pregnancy weight (8), preeclampsia (14), pregnancy complications (12), prenatal testosterone exposure (measured by finger length ratio); 8), perinatal asphyxia (8), labor complications (10), mode of delivery (24), low Apgar score (14), neonatal illness (14), and no breastfeeding (9). For presentation of results, risk factors were categorized as prenatal measures (pre-pregnancy weight, preeclampsia, pregnancy complications, prenatal testosterone exposure), events occurring at birth (perinatal asphyxia, labor complications, mode of delivery), and postnatal measures (Apgar score, neonatal illness, no breastfeeding). Although exposure definitions varied by study, the most common definitions by risk factor are included in Table 2. All studies of testosterone and ADHD were based on measurement of finger length ratio. A lower 2nd digit (2D):4th digit (4D) ratio (2D:4D) has been shown to be associated with elevated prenatal testosterone exposure in both boys and girls (Lutchmaya et al., 2004); thus, negative effect sizes reported for testosterone represent a positive association between testosterone exposure and ADHD.
Table 2.
Results of meta-analyses of studies examining selected prenatal, birth, and postnatal risk factors for attention-deficit/hyperactivity disorder (ADHD)
| Risk factor | Outcome Measure | Most common risk factor definition | ADHD Overall |
ADHD Diagnosis only |
|||
|---|---|---|---|---|---|---|---|
| Total sample size (number of studies) | Pooled effect size (95% CI)* | Total sample size (number of studies) | Pooled effect size (95% CI)* | ||||
| Prenatal risk factors | Pre-pregnancy weight | Dichotomous | Pre-pregnancy body mass index in the obese category (≥ 30 kg/m2) | 1,102,386 (9) | OR: 1.49(1.14; 1.94)** | ||
| Preeclampsia | Dichotomous | Preeclampsia | 972,772 (14) | OR: 1.26 (1.09; 1.46)** | 962,080 (13) | OR: 1.27 (1.10;1.47)** | |
| Pregnancy complications | Dichotomous | Placental abruption most common (others included) | 253,517 (12) | OR: 1.47(1.17; 1.86)** | 226,967 (10) | OR: 1.49 (1.16; 1.91)** | |
| Testosterone | Continuous | Finger length ratio (2nd digit (2D):4D ratio). A lower ratio represents greater testosterone exposure |
1,405 (9) | CC: −0.14 (−0.20; −0.09)** | 526 (3) | CC:−0.16 (−0.25; −0.08)** | |
| Risks occurring at birth | Perinatal asphyxia | Dichotomous | Perinatal asphyxia | 157,051 (8) | OR: 1.31 (0.99; 1.74) | 135,808 (7) | OR: 1.24 (0.92;1.66) |
| Labor complications | Dichotomous | Labor/delivery complications | 139,110 (6) | OR: 1.16(0.92; 1.48) | same as overall | ||
| Continuous | 1,122 (4) | CC: 0.01 (−0.08; 0.11) | |||||
| Mode of delivery | Dichotomous | Breech delivery, cesarean delivery, and vacuum delivery | 2,201,243 (24) | OR: 1.11 (0.99; 1.24) | 1,989,443 (18) | OR: 1.12 (0.98; 1.27) | |
| Breech delivery | Dichotomous | Breech delivery | 247,599 (6) | OR: 1.02 (0.78; 1.34) | 133,183 (3) | OR: 1.03 (0.76–1.41) | |
| Cesarean delivery | Dichotomous | Cesarean delivery | 2,123,517 (22) | OR: 1.12 (1.00; 1.25) | |||
| Vacuum delivery | Dichotomous | Vacuum delivery | 197,957 (5) | OR: 1.10(0.87; 1.39) | |||
| Postnatal risk factors | Apgar score | Dichotomous | Apgar score of <7 at 5 min | 8,189,263 (15) | OR: 1.30 (1.09; 1.54)** | 8,163,236 (13) | OR: 1.29 (1.08; 1.53)** |
| Neonatal illness | Dichotomous | Neonatal intensive care unit (NICU) admission | 166,891 (11) | OR: 1.50 (1.18; 1.92)** | 142,106 (10) | OR: 1.46 (1.14; 1.87)** | |
| Continuous | 913 (3) | CC: 0.11 (0.05; 0.18)** | |||||
| No breastfeeding | Dichotomous | Never breastfed | 13,229 (9) | OR: 1.55 (1.15; 2.10)** | |||
Note: all heterogeneity statistics were not significant and therefore are not reported
Odds ratio (OR) for dichotomous outcomes and correlation coefficient (CC) for continuous outcomes; completed via complete pooling. CI=confidence interval
Significant at p< 0.05
Effect sizes resulting from random-effects meta-analysis for each pregnancy-related risk factor are summarized in Table 2. Forest plots with effect sizes and confidence intervals for individual studies, alongside the summary effect size for each risk factor are presented as supplementary material (see supplemental Figs. 1-25). Heterogeneity statistics (not shown) were non-significant for all analyses, suggesting there was not significant heterogeneity across the studies. Risk factors that were significantly associated with increases in measures of ADHD overall (e.g. all included measures of ADHD) included pre-pregnancy weight, preeclampsia, pregnancy complications, prenatal testosterone exposure, Apgar score, neonatal illness, and lack of breastfeeding. Preeclampsia, pregnancy complications, testosterone, Apgar score, and neonatal illness were also associated with ADHD diagnosis. Testosterone was the only risk factor with at least three effect sizes for measures of inattentive or hyperactive/impulsive symptoms (not presented in Table 2). Higher levels of prenatal testosterone (i.e., lower finger length ratio indicating higher levels of testosterone) was associated with higher levels of inattentive symptoms (six studies; total sample size 1,062; correlation coefficient: −0.16; CI: −0.22, −0.09; p<0.05), as well as hyperactive/impulsive symptoms (seven studies; total sample size 1,139; correlation coefficient: −0.14; CI: −0.20, −0.08; p<0.05). Pre-pregnancy weight, perinatal asphyxia, labor complications, and mode of delivery (including the overall category, as well as breech delivery, Cesarean section, and vacuum delivery), were not significantly associated with ADHD.
Discussion
All prenatal and postnatal risk factors examined had significant positive associations with ADHD. Although risk factors occurring at birth (e.g., mode of delivery) consistently had a positive relationship with ADHD, these factors were not significantly associated with ADHD outcome measures. Notably, the risk factors identified as significantly associated with ADHD tended to represent those with more chronic exposure, rather than acute events such as mode of delivery, or labor complications. This finding aligns with a previous hypothesis that the pregnancy-related factors more frequently associated with ADHD tend to be more chronic in nature, possibly suggesting an increased “dose” of exposure (Milberger et al., 1997). Although neonatal illness is not necessarily chronic, and Apgar score measures infant health immediately at birth, these risk factors may represent poor overall health outcomes and poor neurologic outcomes (Leinonen et al., 2018; Ehrenstein et al., 2009).
The mechanism by which each pregnancy-related factor is associated with ADHD has not been comprehensively investigated and likely varies. In many cases, individual factors might influence neurodevelopment through multiple mechanisms of action. Hypoxia or ischemia can result from preeclampsia or other pregnancy complications (e.g., placental abruption), perinatal asphyxia at birth, and neonatal illness (including respiratory distress syndrome), and might increase the risk for ADHD through impact on the development of the basal ganglia specifically (Ananth et al., 1999; Bos-Veneman et al., 2010; Getahun et al., 2013; Ilekis et al., 2007). Hypoxia may also act more broadly on the brain, as perinatal oxygen deprivation has been shown to be associated with reductions in gray matter volume, intraventricular volume, and periventricular leukomalacia (Getahun et al., 2013). Inflammatory cytokines associated with preeclampsia may influence neural development through inflammatory mechanisms (Silva et al., 2014; Sullivan et al., 2012). The impact of preeclampsia may vary by the timing of onset during pregnancy, and severity of symptoms (Ilekis et al., 2007), factors that were not addressed in the studies included in this meta-analysis.
A recent meta-analysis looking at the association of maternal weight with neurodevelopmental outcomes including ADHD also found an increased risk for ADHD among children whose mothers were overweight or obese prior to pregnancy (Sanchez et al., 2018). Maternal weight may influence child outcomes through multiple pathways. Genetic and environmental risk factors are shared between ADHD and obesity (Faraone et al., 2021). Maternal obesity may influence neurodevelopment through exposure to increased levels of nutrients, hormones, and inflammatory factors (Rivera et al., 2015). In addition, a higher pre-pregnancy BMI is associated with other pregnancy-related risk factors associated with ADHD, including gestational hypertension and preeclampsia, maternal mental health, preterm birth, and neonatal illness, as well as congenital anomalies, possibly through increased risk of gestational diabetes (Mina et al., 2015; Ramachenderan et al., 2008).
The category of pregnancy complications included a wide range of exposures, including excessive vomiting (Bhatia et al., 1991), maternal illness (Pineda et al., 2007), antepartum hemorrhage (Chandola et al., 1992), placental abruption (Motlagh et al., 2010; Getahun et al., 2013), and total number of complications (Kadziela-Olech & Piotrowska-Jastrzebska, 2005). Maternal illness may impact maternal nutrition, and inflammation, both of which can impact early neurodevelopment (Marques et al., 2015). Antepartum hemorrhage and placental abruption may result in oxygen deprivation (Getahun et al., 2013; Walfish et al., 2009) and both are associated with maternal drug use and preterm birth (Ananth et al., 1999; Walfish et al., 2009) which may also be risk factors for ADHD (Vanderbilt & Gleason, 2010; Maher et al., under review in this issue).
Prenatal exposure to increased levels of testosterone may be due to aspects of maternal health, including production by maternal ovaries, elevated levels of insulin (possibly related to obesity or polycystic ovarian syndrome; Lathi et al., 2014), maternal use of anabolic steroids, or exposure to environmental substances with estrogenic or androgenic activity, or in cases of congenital adrenal hyperplasia (Padmanabhan et al., 2006). Elevated prenatal testosterone levels, often indicated by a lower second-digit-to-fourth-digit finger length ratio (2D:4D), have been proposed as a theory for the boys’ elevated risk of ADHD and other neurodevelopmental disorders (de Bruin et al., 2006; Martel et al., 2008). Typically, males have a lower 2D:4D ratio compared to females, and it has been proposed that boys may be more susceptible to elevated levels of testosterone because the central dopamine system has a longer period of development in boys, allowing for increased exposure to elevated hormone levels (Martel et al., 2008; Roberts & Martel, 2013). The analyses presented here included combined estimates across sexes. Future studies could consider the relationship of testosterone on ADHD symptomatology in boys and girls separately.
Although Apgar score is an indicator of the infant’s health status rather than a directly modifiable risk factor, it was included in these analyses because of its widespread use in practice and research. In one analysis of perinatal risk factors for ADHD, a low Apgar score was found to be the most predictive of ADHD, followed by post-term birth (Hanc et al., 2018). Because Apgar score is associated with many pregnancy-related complications (Ehrenstein et al., 2009), it is unlikely to represent a unique risk factor. Interventions and approaches aimed at increasing Apgar scores or other overall indicators of newborn health could be evaluated for potential longer-term impacts on ADHD.
Similar to the categories of pregnancy complications and Apgar score, the “neonatal illness” risk factor category is not specific to a single event or exposure. The neonatal illness category included neonatal or postnatal complications (Ben Amor et al., 2005; Bos-Veneman et al., 2010; Wagner et al., 2009), neonatal intensive care (Froehlich et al., 2009; Hoffman et al., 2010; Sciberras et al., 2011), neonatal resuscitation (Getahun et al., 2013), incubator use (Kim et al., 2009), severe neonatal illness indicated by any hospitalization during the first month of life (Pineda et al., 2007), and whether “the child had any congenital or neurologic problem or any kind of anomaly at birth” (Pires et al., 2013). Both the groups of complications and some of the specific complications included circumstances in which oxygen was limited, or other early stressful events (Ben Amor et al., 2005) that might increase risk for ADHD. Additionally, infants with neonatal illness may have other risk factors for ADHD, such as preterm birth and low birthweight (Wagner et al., 2009).
Findings similar to those reported here, of an increased risk for ADHD among children who were not breastfed, were documented in a meta-analysis focused exclusively on breastfeeding that included only one overlapping article with the analyses presented here (Tseng et al., 2019). The association of a lack of breastfeeding with ADHD might be related to multiple mechanisms including nutritional factors, hormone exposure, immunity transfer, as well as social factors (Silva et al., 2014; Tseng et al., 2019). In addition, breastfeeding is related to improved maternal-child attachment which is associated with improved attention (Hayatbakhsh et al., 2012), and with reductions in child maltreatment (Hayatbakhsh et al., 2012) and maternal depression (Dias & Figueiredo, 2015) which are both risk factors for ADHD (Claussen et al., this issue; Robinson et al., this issue). Admission to the NICU is also associated with decreased breastfeeding (Maia et al., 2011) and increased risk for ADHD (Chiorean et al., 2020). The sensory experience associated with breastfeeding has also been proposed as a mechanism for improving cognitive development (Mimouni-Bloch et al., 2013). The studies included in the meta-analysis reported here reported on breastfeeding and did not include a group of children provided breastmilk in the absence of breastfeeding; thus, the mechanism contributing to these findings cannot be determined.
Only factors occurring at the time of birth were not associated with increased risk for ADHD including labor complications, mode of delivery, and perinatal asphyxia. Although asphyxia has been shown to have a wide-range of effects on neurodevelopment, including cognitive impairments, no significant association was found for perinatal asphyxia and ADHD. There may be multiple causes of asphyxia but the timing, severity, and causes of asphyxia were not analyzed as specific factors in this study. Although asphyxia can be severe, it can also be short in duration, and may be associated with more select damage to the brain that might be more easily repaired (Korkman et al., 1994). One author suggested that asphyxia may be associated with either severe damage, where the children might be excluded from the study because of very low Intelligence Quotient (IQ), or minimal or no neural damage, which would be more likely in children included in the research protocols (Korkman et al., 1994). In fact, of the five studies of perinatal asphyxia included in the meta-analysis, two excluded children based on their IQ (Motlagh et al., 2010; Pineda et al., 2007), and two excluded children with autism spectrum disorder or pervasive developmental disorder (Getahun et al., 2013; Pringsheim et al., 2009). Furthermore, more severe asphyxia, or prolonged complications associated with asphyxia, may have been captured in the categories of Apgar score, and neonatal illness, which was often characterized by receiving care in the neonatal intensive care unit (NICU). Of note, preeclampsia may cause chronic exposure to asphyxia-type conditions (Ilekis et al., 2007), and was associated with ADHD in these meta-analyses. Like asphyxia, labor complications and mode of delivery are relatively acute events that may restrict oxygen delivery to the baby, potentially impacting the brain. However, neither of these, including specific modes of delivery (e.g., breech), were associated with ADHD outcomes.
At least five limitations are associated with these meta-analyses. First, as with all studies based on published literature, these results cannot be assumed to generalize beyond the populations in the included studies. Second, despite conducting the literature search with an intent to capture all relevant articles, the findings are not comprehensive of all potential risk factors. Some risk factors may have been missed by our search strategies, and some articles and risk factors were excluded due to insufficient data for the meta-analysis (e.g., fewer than three articles on maternal infection and ADHD were identified) or resource limitations for the overall project. Since the initial review in 2014, there may be new studies of additional risk factors, including maternal autoimmune disease, that may be associated with ADHD (Nielsen et al., 2021). Third, many of the categories of risk factors were broad, some contained a variety of more specific exposures (including some for which evidence on modifiability is still lacking), and there were a variety of measures of association types reported. In particular, the categories of pregnancy complications and neonatal illness each included a number of different exposures, which potentially occurred at different times during pregnancy and the neonatal period. Future research could examine the association of more specific risk factors (e.g., maternal diabetes, neonatal hypothermia) or timing of exposure with ADHD outcomes. Fourth, although there is a strong genetic component to ADHD, and evidence for gene-environment interaction, genetic factors were beyond the scope of these analyses. Incidentally, one study identified perinatal complications as nonshared environmental factors that increased risk of ADHD in individuals compared with their siblings (Ben Amor et al., 2005). Future studies can include other related and potentially confounding factors such as child age, sex, and other parental factors including family history of ADHD. Fifth, many of the pregnancy-related risk factors examined are associated both with each other, (Ehrenstein et al., 2009) and with other identified risk factors for ADHD, including maternal mental health (Blom et al., 2010; Robinson et al., this issue), and parental substance use (Maher et al., under review in this issue). Our analytic approach examined risk factors separately (vs. multi-level modeling) to ensure that findings across risk factors and papers could be meaningfully compared. Future research could include multi-level modeling to better understand the relationships between risk factors.
Despite these limitations, the findings reported here highlight the association between prenatal and postnatal risk factors and ADHD. Each of the pooled odds ratios for the prenatal and postnatal factors was between 1.3 and 1.5; the corresponding range for pooled correlation coefficients was −0.16–0.11. These findings represent small, but significant, effect sizes (Chen et al., 2010; Cohen, 1988), and agree with the finding of Faraone et al. (2021) that ADHD is most often caused by the combined effect of multiple risk factors, with each contributing a small risk. Although the prevalence of pregnancy-related risk factors associated with ADHD vary widely, the most common include pregnancy weight, with approximately 50% of mothers being overweight or obese prior to pregnancy (Jo et al., 2015), pregnancy complications, present in approximately half (46.9%) of pregnancies (Ananth et al., 2013), lack of breastfeeding among about 16% of infants (Centers for Disease Control and Prevention, 2020), and NICU admittance for 12% of newborns (U.S. Department of Health and Human Services, 2013). Less common pregnancy-related risk factors include preeclampsia, which occurs in approximately 3% of pregnancies (Ananth et al., 2013), and a low Apgar score (< 7), present in less than one percent of infants (Li et al., 2013). The prevalence for testosterone exposure is less certain. Studies examining the effect of testosterone on ADHD diagnosis and symptoms used the 2D:4D finger ratio as a proxy for testosterone exposure. We are not aware of an established threshold for this ratio or an associated prenatal testosterone exposure level that could be used to provide a prevalence estimate. Given the prevalence of the pregnancy-associated factors found to be associated with ADHD, and the impact of ADHD, and the long-term association of perinatal risk factors with ADHD symptoms and associated outcomes (Tervo et al., 2017) these factors may serve as potential targets for preventing or mitigating the symptoms of ADHD, and to inform future research to better understand more specific factors (e.g. specific modifiable pregnancy complications).
In addition to the association of specific pre- and postnatal risk factors and ADHD, these risk factors may be more broadly associated with neurodevelopment and other related disorders. For example, pre-pregnancy maternal weight has been shown to be associated with autism spectrum disorder, developmental delay, emotional/behavioral problems, and cognitive delay (Sanchez et al., 2018). Pregnancy complications including preeclampsia have been shown to be associated with increased risk for autism spectrum disorder (ASD) and other developmental delays (Walker et al., 2015), and exposure to elevated prenatal levels of testosterone have also been shown to be associated with ASD, total behavioral difficulties, and conduct problems (de Bruin et al., 2006; Fink et al., 2007). Low Apgar scores have been shown to be associated with cerebral palsy, epilepsy, intellectual disability, and sensorineural deficits (Leinonen et al., 2018). Breastfeeding has been associated with reduced problem behaviors including social problems, aggressive behaviors (Hayatbakhsh et al., 2012), as well as motor development (Sacker et al., 2006). Thus, prevention activities to reduce these risk factors may have greater impact than only reducing ADHD. Furthermore, the pregnancy-related risk factors examined here are also associated with maternal health, supporting prenatal care, neonatal care, and breastfeeding.
Attention to prenatal and maternal health and neonatal care is fundamental to establishing the foundation for lifelong physical and mental health (Brundage & Shearer, 2019). Optimizing maternal and child health can be challenging due to somewhat independent systems serving women (e.g., obstetrics) and children (e.g., pediatrics). Improving both maternal and child health could be facilitated through an “integrated family care” approach (Brundage & Shearer, 2019). In addition to promoting maternal health and prenatal care, an integrated approach could allow for sharing of health information from the prenatal and early neonatal period with pediatricians. In this way, the identified prenatal and early postnatal risk factors could inform future screening and prevention efforts to improve outcomes related to ADHD and neurodevelopment more generally. Early identification of infants at risk for ADHD and other neurodevelopmental disorders may improve the time to referral for intervention services and could improve outcomes among children at risk for these disorders.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Lu (Mary) Meng, Ph.D., and Jaleal Sanjak, Ph.D. for their assistance in creating the Forest Plots for these analyses.
The findings and conclusions in this manuscript are those of the authorsand do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding
National Center on Birth Defects and Developmental Disabilities,ID04130157.
Footnotes
The work presented here was completed through an Interagency agreement between the Centers for Disease Control and Prevention and the General Service Administration (13-FED-1303304). The work was completed under GSA Order Number ID04130157 to Gryphon Scientific, LLC, titled “Identifying Public Health Strategies with Potential for Reducing Risk for Attention-Deficit/Hyperactivity Disorder.”
Declarations
Conflict of Interest All authors report no potential conflicts of interest.
Research Involving Human Participants and/or Animals This study includes analyses of data previously published in the literature.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/sll121-022-01359-3.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5). American Psychiatric Publishing. 10.l176/appi.books.9780890425596 [DOI] [Google Scholar]
- Ananth CV, Berkowitz GS, Savitz DA, & Lapinski RH (1999). Placental abruption and adverse perinatal outcomes. Journal of the American Medical Association, 282(17), 1646–1651. 10.1001/jama.282.17.1646 [DOI] [PubMed] [Google Scholar]
- Ananth CV, Keyes KM, & Wapner RJ (2013). Pre-eclampsia rates in the United States, 1980–2010: Age-period-cohort analysis. British Medical Journal, 347, f6564. 10.1136/bmj.f6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Amor L, Grizenko N, Schwartz G, Lageix P, Baron C, Ter-Stepanian M, Zappitelli M, Mbekou V, & Joober R. (2005). Perinatal complications in children with attention-deficit hyperactivity disorder and their unaffected siblings. Journal of Psychiatry & Neuroscience: JPN, 30, 120–126. [PMC free article] [PubMed] [Google Scholar]
- Berlin JA, Laird NM, Sacks HS, & Chalmers TC (1989). A comparison of statistical methods for combining event rates from clinical trials. Statistics and Medicine, 8, 141–151. 10.1002/sim.4780080202 [DOI] [PubMed] [Google Scholar]
- Bhatia MS, Nigam VR, Bohra N, & Malik SC (1991). Attention deficit disorder with hyperactivity among paediatric outpatients. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 32, 297–306. 10.llll/j.1469-7610.1991.tb00308.x [DOI] [PubMed] [Google Scholar]
- Blom EA, Jansen PW, Verhulst EC, Hofman A, Raat H, Jaddoe VW, Coolman M, Steegers EA, & Tiemeier H. (2010). Perinatal complications increase the risk of postpartum depression. The Generation R Study. BJOG: An International Journal of Obstetrics and Gynaecology, 117(11), 1390–1398. 10.1111/j.1471-0528.2010.02660.X [DOI] [PubMed] [Google Scholar]
- Böhm S, Curran EA, Kenny LC, O’Keeffe GW, Murray D, & Khashan AS (2019). The effect of hypertensive disorders of pregnancy on the risk of ADHD in the offspring. Journal of Attention Disorders, 23, 692–701. 10.1177/1087054717690230 [DOI] [PubMed] [Google Scholar]
- Bos-Veneman NGP, Kuin A, Minderaa RB, & Hoekstra PJ (2010). Role of perinatal adversities on tic severity and symptoms of attention deficit/hyperactivity disorder in children and adolescents with a tic disorder. Journal of Developmental and Behavioral Pediatrics, 31, 100–106. 10.1097/DBP.0b013e3181cc7cbc [DOI] [PubMed] [Google Scholar]
- Brion MJ, Zeegers M, Jaddoe V, Verhulst F„ Tiemeier H, Lawlor DA, & Smith GD (2011). Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics, 127, e202–211. 10.1542/peds.2010-0651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage SC, & Shearer C. (2019). Plan and Provider Opportunities to Move Toward Integrated Family Health Care. March 21. United Hospital Fund, pp. 1–13. Available at: https://uhfnyc.org/media/filer_public/cl/0b/cl0bfb65-dbf9-4d2c-9f97-ecd2a3928a9e/plan_and_provider_opportunities_uhf.pdf. Accessed 6 May 2021.
- Centers for Disease Control and Prevention. (2020). Breastfeeding Report Card. https://www.cdc.gov/breastfeeding/pdiy2020-Breastfeeding-Report-Card-H.pdf. Accessed 6 May 2020.
- Chandola CA, Robling MR, Peters TJ, Melville-Thomas G, & McGuffln P. (1992). Pre- and perinatal factors and the risk of subsequent referral for hyperactivity. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 33,1077–1090. 10.l111/j.1469-7610.1992.tb00926.x [DOI] [PubMed] [Google Scholar]
- Chen H, Cohen P, & Chen S. (2010). How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Communications in Statistics - Simulation and Computation, 39, 860–864. 10.1080/03610911003650383 [DOI] [Google Scholar]
- Chen MH„ Pan TL„ Wang PW„ Hsu JW„ Huang KL„ Su TP, Li CT„ Lin WC„ Tsai SJ, Chen TJ, & Bai YM (2019). Prenatal exposure to acetaminophen and the risk of attention-deficit/hyperactivity disorder: A nationwide study in Taiwan. The Journal of Clinical Psychiatry, 80(5). 10.4088/JCP.18ml2612 [DOI] [PubMed] [Google Scholar]
- Chiorean A, Savoy C, Beattie K, El Helou S, Silmi M, & Van Lieshout RJ (2020). Childhood and adolescent mental health of NICU graduates: an observational study. Archives of disease in childhood, Published online 23 Jan 2020. 10.1136/archdischild-2019-318284 [DOI] [PubMed]
- Claussen AH, Holbrook JR, Hutchins H, Robinson LR, Bloomfield J, Meng L, Bitsko RH, O’Masta B, Cerles A, Maher B, Rush M, & Kaminski JW (this issue). All in the family? A systematic review and meta-analysis of parenting and family environment as risk factors for attention-deficit/hyperactivity disorder (ADHD) in children. Prevention Science. [DOI] [PMC free article] [PubMed]
- Claycomb CD, Ryan JJ, Miller LJ, & Schnakenberg-Ott SD (2004). Relationships among attention deficit hyperactivity disorder, induced labor, and selected physiological and demographic variables. Journal of Clinical Psychology, 60, 689–693. 10.1002/jclp.10238 [DOI] [PubMed] [Google Scholar]
- Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, Erb JL, Churchill SE, Kaimal AJ, Doyle AE, Robinson EB, Smoller JW, Kohane IS, & Perlis RH (2015). Prenatal anti-depressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Molecular Psychiatry, 20,727–734. 10.1038/mp.2014.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR (1988). Statistical Power Analysis for the Behavioral Sciences. Routledge. [Google Scholar]
- Curran EA, Khashan AS, Dalman C, Kenny LC, Cryan JF, Dinan TG, & Kearney PM (2016). Obstetric mode of delivery and attention-deficit/hyperactivity disorder: A sibling-matched study. International Journal of Epidemiology, 45,532–542. 10.1093/ije/dyw001 [DOI] [PubMed] [Google Scholar]
- D’Souza S, Waldie KE, Peterson ER, Underwood L, & Morton SMB (2019). Antenatal and postnatal determinants of behavioural difliculties in early childhood: Evidence from Growing Up in New Zealand. Child Psychiatry and Human Development, 50, 45–60. 10.1007/sl0578-018-0816-6 [DOI] [PubMed] [Google Scholar]
- Dachew BA, Scott JG, Mamun A, & Alati R. (2019). Preeclampsia and the risk of attention-deficit/hyperactivity disorder in offspring: Findings from the ALSPAC birth cohort study. Psychiatry Research, 272, 392–397. 10.1016/j.psychres.2018.12.123 [DOI] [PubMed] [Google Scholar]
- Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, & Blumberg SJ (2018). Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. Journal of Clinical Child & Adolescent Psychology, 47(2), 199–212. 10.1080/15374416.2017.1417860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin E, Verheij F, Wiegman T, & Ferdinand R. (2006). Differences in finger length ratio between males with autism, pervasive developmental disorder-not otherwise specified, ADHD, and anxiety disorders. Developmental Medicine and Child Neurology, 48,962–965. 10.1017/S0012162206002118 [DOI] [PubMed] [Google Scholar]
- Dean RS, & Davis AS (2007). Relative risk of perinatal complications in common childhood disorders. School Psychology Quarterly, 22,13–25. 10.1037/1045-3830.22.l.13 [DOI] [Google Scholar]
- DerSimonian R, & Laird N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7, 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Dias CC, & Figueiredo B. (2015). Breastfeeding and depression: A systematic review of the literature. Journal of Affective Disorders, 171,142–154. 10.1016/j.jad.2014.09.022 [DOI] [PubMed] [Google Scholar]
- Dimitrov LV, Kaminski JW, Holbrook JR, Bitsko RH, Yeh M, O’Masta B,Maher B, Cerles A, & Rush M. (under review in this issue). A systematic review and meta-analysis of chemical exposures and attention-deficit, hyperactivity disorder. Prevention Science. Ehrenstein V, Pedersen L, Grijota M, Nielsen GL, Rothman KJ, & Sorensen HT (2009). Association of Apgar score at five minutes with long-term neurologic disability and cognitive function in a prevalence study of Danish conscripts. BMC Pregnancy and Childbirth, 9,14. 10.1186/1471-2393-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein V, Pedersen L, Grijota M, Nielsen GL, Rothman KJ, & Sorensen HT (2009). Association of Apgar score at five minutes with long-term neurologic disability and cognitive function in a prevalence study of Danish conscripts. BMC Pregnancy and Childbirth, 9, 14. 10.1186/1471-2393-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine HE, Norman RE, Ferrari AJ, Chan GC, Copeland WE, Whiteford HA, & Scott JG (2016). Long-term outcomes of attention-deficit/hyperactivity disorder and conduct disorder: A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 841–850. 10.1016/j.jaac.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, Newcom JH, Gignac M, Al Saud NM, Manor I, Rohde LA, Yang L, Cortese S, Almagor D, Stein M, A., Albatti TH, Aljoudi HF, Alqahtani MMJ, Asherson P, & Wang Y. (2021). The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neuroscience & Biobehavioral Reviews. Online ahead of print 4 Feb 2021. 10.1016/j.neubiorev.2021.01.022 [DOI] [PMC free article] [PubMed]
- Fianu S, & Joelsson I. (1979). Minimal brain dysfunction in children bom in breech presentation. Acta Obstetricia Et Gyneco-logica Scandinavica, 58, 295–299. 10.3109/00016347909154052 [DOI] [PubMed] [Google Scholar]
- Fink B, Manning J, Williams J, & Podmore-Nappin C. (2007). The 2nd to 4th digit ratio and developmental psychopathology in school-aged children. Personality and Individual Differences, 42, 369–379. 10.1016/j.paid.2006.07.018 [DOI] [Google Scholar]
- Froehlich TE, Lanphear BP, Auinger P, Hornung R, Epstein JN, Braun J, & Kahn RS (2009). Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics, 124, el054–1063. 10.1542/peds.2009-0738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Anixt JS, Loe IM, Chirdkiatgumchai V, Kuan L, & Gilman RC (2011). Update on environmental risk factors for attention-deficit/hyperactivity disorder. Current Psychiatry Reports, 13, 333–344. 10.1007/si1920-011-0221-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getahun D, Rhoads GG, Demissie K, Lu SE, Quinn VP, Fassett MJ, Wing DA, & Jacobsen SJ (2013). In utero exposure to ischemic-hypoxic conditions and attention-deficit/hyperactivity disorder. Pediatrics, 131, e53–61. 10.1542/peds.2012-1298 [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, lams JD, & Romero R. (2008). Epidemiology and causes of preterm birth. Lancet, 371, 75–84. 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhn M, Emerson SD, Mahdaviani D, & Gadermann AM (2020). Associations of birth factors and socio-economic status with indicators of early emotional development and mental health in childhood: A population-based linkage study. Child Psychiatry and Human Development, 51,80–93. 10.1007/sl0578-019-00912-6 [DOI] [PubMed] [Google Scholar]
- Gurevitz M, Geva R, Varon M, & Leitner Y. (2014). Early Markers in Infants and Toddlers for Development of ADHD. Journal of Attention Disorders, 18,14–22. 10.1177/1087054712447858 [DOI] [PubMed] [Google Scholar]
- Gustafsson R, & Kallen K. (2011). Perinatal, maternal, and fetal characteristics of children diagnosed with attention-deficit-hyperactivity disorder: results from a population-based study utilizing the Swedish Medical Birth Register. Developmental Medicine and Child Neurology, 55(3), 263–268. 10.llll/j.1469-8749.2010.03820.x [DOI] [PubMed] [Google Scholar]
- Gustavson K, Ask H, Ystrom E, Stoltenberg C, Lipkin WI, Surén P, Håberg SE, Magnus P, Knudsen GP, Eilertsen E, Bresnahan M, Aase H, Mjaaland S, Susser ES, Hornig M, & Reichborn-Kjennerud T. (2019). Maternal fever during pregnancy and offspring attention deficit hyperactivity disorder. Scientific Reports, 9, 9519. 10.1038/s41598-019-45920-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JY„ Kwon HJ, Ha M„ Paik KC„ Lim MH„ Gyu Lee S, Yoo SJ, & Kim EJ (2015). The effects of prenatal exposure to alcohol and environmental tobacco smoke on risk for ADHD: A large population-based study. Psychiatry Research, 225,164–168. 10.1016/j.psychres.2014.ll.009 [DOI] [PubMed] [Google Scholar]
- Hanć T, Szwed A, Slopien A, Wolanczyk T, Dmitrzak-Weglarz M, & Ratajczak J. (2018). Perinatal risk factors and ADHD in children and adolescents: a hierarchical structure of disorder predictors. Journal of Attention Disorders, 22, 855–863. 10.1177/1087054716643389 [DOI] [PubMed] [Google Scholar]
- Hayatbakhsh MR, O’Callaghan MJ, Bor W, Williams GM, & Najman JM (2012). Association of breastfeeding and adolescents’ psychopathology: A large prospective study. Breastfeeding Medicine, 7, 480–486. 10.1089/bfm.2011.0136 [DOI] [PubMed] [Google Scholar]
- Hedges LV, & Olkin I. (1985). Statistical methods for meta-analysis. Academic Press. [Google Scholar]
- Hoffman K, Webster TF, Weisskopf MG, Weinberg J, & Vieira VM (2010). Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age. Environmental Health Perspectives, 118(12), 1762–1767. 10.1289/ehp.1001898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilekis JV, Reddy UM, & Roberts JM (2007). Preeclampsia-a pressing problem: An executive summary of a National Institute of Child Health and Human Development workshop. Reproductive Sciences, 14,508–523. 10.1177/1933719107306232 [DOI] [PubMed] [Google Scholar]
- Instanes JT, Halmoy A, Engeland A, Haavik J, Furu K, & Klungsoyr K. (2017). Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biological Psychiatry, 81,452–459. 10.1016/j.biopsych.2015.11.024 [DOI] [PubMed] [Google Scholar]
- Ji Y, Hong X, Wang G, Chatterjee N, Riley AW, Lee LC, Surkan PJ, Bartell TR, Zuckerman B, & Wang X. (2018). A prospective birth cohort study on early childhood lead levels and attention deficit hyperactivity disorder: New insight on sex differences. The Journal of Pediatrics, 199, 124–131.e8. 10.1016/j.jpeds.2018.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Du Y, Zhong X, & David C. (2014). Prevalence and contributing factors to attention deficit hyperactivity disorder: A study of five- to fifteen-year-old children in Zhabei District. Shanghai Asia- Pacific Psychiatry, 6, 397–404. 10.llll/appy.12114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H, Schieve LA, Sharma AJ, Hinkle SN, Li R, & Lind JN (2015). Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics, 135, el198–el209. 10.1542/peds.2014-3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joelsson P, Chudal R, Talati A, Suominen A, Brown AS, & Sourander A. (2016). Prenatal smoking exposure and neuropsychiatric comorbidity of ADHD: A furnish nationwide population-based cohort study. BMC Psychiatry, 16, 306. 10.1186/S12888-016-1007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julvez J, Ribas-Fito N, Forns M, Garcia-Esteban R, Torrent M, & Sunyer J. (2007). Attention behaviour and hyperactivity at age 4 and duration of breastfeeding. Acta Paediatrica, 96,842–847. 10.1111/j.1651-2227.2007.00273.x [DOI] [PubMed] [Google Scholar]
- Kadziela-Olech H, & Piotrowska-Jastrzebska J. (2005). The duration of breastfeeding and attention deficit hyperactivity disorder. Roczniki Akademii Medycznej w Biatymstoku, 1995, 302–306. 10.1080/24694193.2020.1797236 [DOI] [PubMed] [Google Scholar]
- Källén AJ, Finnstrom OO, Lindam AP, Nilsson EM, Nygren KG, & Otterblad Olausson PM (2011). Is there an increased risk for drug treated attention deficit/hyperactivity disorder in children born after in vitro fertilization? European Journal of Pediatrics Neurol, 15, 247–253. 10.1016/j.ejpn.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Kim HW„ Cho SC„ Kim BN„ Kim JW„ Shin MS, & Kim Y. (2009). Perinatal and familial risk factors are associated with full syndrome and subthreshold attention-deficit hyperactivity disorder in a Korean community sample. Psychiatry Investigation, 6(4), 278–285. 10.4306/pi.2009.6.4.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Hilakivi-Clarke LA, Autti-Rämö I, Fellman V, & Granström ML (1994). Cognitive impairments at two years of age after prenatal alcohol exposure or perinatal asphyxia. Neuropediatrics, 25,101–105. 10.1055/s-2008-1071594 [DOI] [PubMed] [Google Scholar]
- Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C, & Gardner RM (2017). Maternal polycystic ovary syndrome and risk for attention-deficil/hyperactivity disorder in the offspring. Biological Psychiatry, 82, 651–659. 10.1016/j.biopsych.2016.09.022 [DOI] [PubMed] [Google Scholar]
- Lathi RB, Dahan MH, Reynolds-May MF, Milki AA, Behr B, & Westphal LM (2014). The role of serum testosterone in early pregnancy outcome: A comparison in women with and without polycystic ovary syndrome. Journal of Obstetrics and Gynaecology Canada, 36, 811–816. 10.1016/S1701-2163(15)30483-7 [DOI] [PubMed] [Google Scholar]
- Leinonen E, Gissler M, Haataja L, Rahkonen P, Andersson S, Metsaranta M, & Rahkonen L. (2018). Low Apgar scores at both one and five minutes are associated with long-term neurological morbidity. Acta Paediatrica, 107, 942–951. 10.1111/apa.l4234 [DOI] [PubMed] [Google Scholar]
- Li F„ Wu T„ Lei X, Zhang H„ Mao M„ & Zhang J. (2013). The apgar score and infant mortality. PLoS One, 8, e69072. 10.137l/journal.pone.0069072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, & Obel C. (2011). Low Apgar scores and risk of childhood attention deficit hyperactivity disorder. The Journal of Pediatrics, 158, 775–779. 10.1016/j.jpeds.2010.10.041 [DOI] [PubMed] [Google Scholar]
- Lin Q„ Hou XY„ Yin XN„ Wen GM„ Sun D„ Xian DX, Fan L„ Jiang H„ Jing J, Jin Y„ Wu C„ & Chen W. (2017). Prenatal exposure to environmental tobacco smoke and hyperactivity behavior in Chinese young children. International Journal of Environmental Research and Public Health, 14(10). 10.3390/ijerphl4101132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet KM, Obel C, Bonde E, Hove Thomsen P, Secher NJ, Wisborg K, & Brink Henriksen T. (2006). Cigarette smoking during pregnancy and hyperactive-distractible preschooler’s: A follow-up study. Acta Paediatrica, 95, 694–700. 10.1080/08035250500459709 [DOI] [PubMed] [Google Scholar]
- Liu J, Portnoy J, & Raine A. (2012). Association between a marker for prenatal testosterone exposure and externalizing behavior problems in children. Development and Psychopathology, 24, 771–782. 10.1017/S0954579412000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley. (2018). Package ‘rmeta.’ Version 3.0. https://cran.r-project.org/web/packages/rmeta/rmeta.pdf. Accessed 13 Mar 2019.
- Lutehmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, & Manning JT (2004). 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Human Development, 77, 23–28. 10.1016/j.earlhumdev.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Maher B, Kaminski J, O’Masta B, Cerles A, Holbrook JR, Mahmooth Z, & Rush M. (under review in this issue). A systematic meta-analysis of the relationship between exposure to parental substance use and attention-deficit/hyperactivity disorder. Prevention Science. [DOI] [PMC free article] [PubMed]
- Maher GM, O’Keeffe GW, O’Keeffe LM, Matvienko-Sikar K, Dalman C, Kearney PM, McCarthy FP, & Khashan AS (2020). The association between preeclampsia and childhood development and behavioural outcomes. Maternal and Child Health Journal, 24, 727–738. 10.1007/s10995-020-02921-7 [DOI] [PubMed] [Google Scholar]
- Maia C, Brandao R, Roncalli A, & Maranhao H. (2011). Length of stay in a neonatal intensive care unit and its association with low rates of exclusive breastfeeding in very low birth weight infants. The Journal of Maternal-Fetal & Neonatal Medicine, 24, 774–777. 10.3109/14767058.2010.520046 [DOI] [PubMed] [Google Scholar]
- Mann JR, & McDermott S. (2011). Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? Journal of Attention Disorders, 15, 667–673. 10.1177/1087054710370566 [DOI] [PubMed] [Google Scholar]
- Marques AH, Bjorke-Monsen AL, Teixeira AL, & Silverman MN (2015). Maternal stress, nutrition and physical activity: Impact on immune function, CNS development and psychopathology. Brain Research, 1617,28–46. 10.1016/j.brainres.2014.10.051 [DOI] [PubMed] [Google Scholar]
- Martel M. (2009). Conscientiousness as a mediator of the association between masculinized finger-length ratios and attention-deficit/hyperactivity disorder (ADHD). Journal of Child Psychology and Psychiatry, and Allied Disciplines, 50,790–798. 10.1111/j.1469-7610.2009.02065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel M, Gobrogge K, Breedlove S, & Nigg J. (2008). Masculinized finger-length ratios of boys, but not girls, are associated with attention-deficit/hyperactivity disorder. Behavioral Neuroscience, 122, 273–281. 10.1037/0735-7044.122.2.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Westhafer J, Pasanen E, Carlson C, & Tucker D. (2005). Physiological evidence of hypermasculinization in boys with the inattentive type of attention-deficit/hyperactivity disorder (ADHD). Clinical Neuroscience Research, 5, 233–245. 10.1016/j.cnr.2005.09.004 [DOI] [Google Scholar]
- McIntosh DE, Mulkins RS, & Dean RS (1995). Utilization of maternal perinatal risk indicators in the differential diagnosis of ADHD and UADD children. The International Journal of Neuroscience, 81, 35–46. 10.3109/00207459509015297 [DOI] [PubMed] [Google Scholar]
- Melchior M, Hersi R, van der Waerden J, Larroque B, Saurel-Cubizolles MJ, Chollet A, Galèra C, & the EDEN Mother-Child Cohort Study Group. (2015). Maternal tobacco smoking in pregnancy and children’s socio-emotional development at age 5: The EDEN mother-child birth cohort study. European Psychiatry, 30, 562–568. 10.1016/j.eurpsy.2015.03.005 [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Guite J, & Tsuang MT (1997). Pregnancy, delivery and infancy complications and attention deficit hyperactivity disorder: issues of gene-environment interaction. Biological psychiatry, 41, 65–75. 10.1016/0006-3223(95)00653-2 [DOI] [PubMed] [Google Scholar]
- Mimouni-Bloch A, Kachevanskaya A, Mimouni FB, Shuper A, Raveh E, & Linder N. (2013). Breastfeeding may protect from developing attention-deflcit/hyperactivity disorder. Breastfeeding Medicine, 8, 363–367. 10.1089/bfm.2012.0145 [DOI] [PubMed] [Google Scholar]
- Mina TH, Denison FC, Forbes S, Stirrat LI, Norman JE, & Reynolds RM (2015). Associations of mood symptoms with ante- and postnatal weight change in obese pregnancy are not mediated by cortisol. Psychological Medicine, 45, 3133–3146. 10.1017/S0033291715001087 [DOI] [PubMed] [Google Scholar]
- Motlagh MG, Katsovich L, Thompson N, Lin H, Kim YS, Scahill L, et al. (2010). Severe psychosocial stress and heavy cigarette smoking during pregnancy An examination of the pre- and perinatal risk factors associated with ADHD and Tourette syndrome. European Child & Adolescent Psychiatry, 19, 755–764. 10.1007/s00787-010-0115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, Pearson R, Fernandes M, Santos IS, Barros FC, Victora CG, Stein A, & Matijasevich A. (2016). Are fetal growth impairment and preterm birth causally related to child attention problems and ADHD? Evidence from a comparison between high-income and middle-income cohorts. Journal of Epidemiology and Community Health, 70,704–709. 10.1136/jech-2015-206222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TC, Nassar N, Shand AW, Jones H, Guastella AJ, Dale RC, & Lain SJ (2021). Association of maternal autoimmune disease with attention-deficit/hyperactivity disorder in children. JAMA Pediatrics, 175, e205487. 10.lOOl/jamapediatrics.2020.5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Manikkam M, Recabarren S, & Foster D. (2006). Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Molecular and Cellular Endocrinology, 246,165–174. 10.1016/j.mce.2005.ll.016 [DOI] [PubMed] [Google Scholar]
- Park S, Cho SC„ Kim JW„ Shin MS, Yoo HJ, Oh SM„ Han DH„ Cheong JH„ & Kim BN (2014a). Differential perinatal risk factors in children with attention-deficit/hyperactivity disorder by subtype. Psychiatry Research, 219, 609–616. 10.1016/j.psychres.2014.05.036 [DOI] [PubMed] [Google Scholar]
- Park S, Kim BN„ Kim JW„ Shin MS, Yoo HJ, & Cho SC (2014b). Protective effect of breastfeeding with regard to children’s behavioral and cognitive problems. Nutrition Journal, 13, 111. 10.1186/1475-2891-13-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda DA, Palacio LG, Puerta IC, Merchan V, Arango CP, Galvis AY, G6mez M, Aguirre DC, Lopera F, & Arcos-Burgos M. (2007). Environmental influences that affect attention deficit/hyperactivity disorder: Study of a genetic isolate. European Child & Adolescent Psychiatry, 16, 337–346. 10.1007/s00787-007-0605-4 [DOI] [PubMed] [Google Scholar]
- Pires TDO, da Silva CMFP, & de Assis SG (2013). Association between family environment and attention deficit hyperactivity disorder in children-mothers’ and teachers’ views. BMC psychiatry, 13(1), 1–9. 10.1186/1471-244X-13-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlabeln H, Rach S, De Henauw S, Eiben G, Gwozdz W, Hadjigeorgiou C, Molnár D, Moreno LA, Russo P, Veidebaum T, & Pigeot I. (2017). Further evidence for the role of pregnancy-induced hypertension and other early life influences in the development of ADHD: Results from the IDEFTCS study. European Child & Adolescent Psychiatry, 26, 957–967. 10.1007/s00787-017-0966-2 [DOI] [PubMed] [Google Scholar]
- Pringsheim T, Sandor P, Lang A, Shah P, & O’Connor P. (2009). Prenatal and perinatal morbidity in children with Tourette syndrome and attention-deficit hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics, 30,115–121. 10.1097/DBP.0b013e31819e6a33 [DOI] [PubMed] [Google Scholar]
- Ramachenderan J, Bradford J, & McLean M. (2008). Maternal obesity and pregnancy complications: A review. The Australia and New Zealand Journal of Obstetrics and Gynaecology, 48, 228–235. 10.llll/j.1479-828X.2008.00860.x [DOI] [PubMed] [Google Scholar]
- Rice D, & Barone S Jr. (2000). Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environmental Health Perspectives, 108, 511–533. 10.1289/ehp.00108s3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera HM, Christiansen KJ, & Sullivan EL (2015). The role of maternal obesity in the risk of neuropsychiatric disorders. Frontiers in Neuroscience, 9,194. 10.3389/fnins.2015.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B, & Martel M. (2013). Prenatal testosterone and preschool Disruptive Behavior Disorders. Personality and Individual Differences, 55(8), 962–966. 10.1016/j.paid.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LR, Bitsko RH, O’Masta B, Holbrook JR, Ko J, Maher B, Cerles A, Saadeh K, MacMillan L, Mahmooth Z, Bloomfield J, Rush M, & Kaminski J. (this issue). A Systematic review and meta-analysis of parental depression, anti-depressant usage, antisocial personality disorder, and stress and anxiety as risk factors for attention-deficit/hyperactivity disorder (ADHD) among children. Prevention Science. [DOI] [PMC free article] [PubMed]
- Rodriguez A. (2010). Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. Journal of Child Psychology and Psychiatry, 51(2), 134–143. 10.1111/j.l469-7610.2009.02133.x [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Miettunen J, Henriksen TB, Olsen J, Obel C, Taanila A, Ebeling H, Linnet KM, Moilanen I, & Järvelin MR (2008). Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. International Journal of Obesity (2005), 32(3), 550–557. 10.1038/sj.ijo.0803741 [DOI] [PubMed] [Google Scholar]
- Russell G, Rodgers LR, Ukoumunne OC, & Ford T. (2014). Prevalence of parent-reported ASD and ADHD in the UK: Findings from the Millennium Cohort Study. Journal of Autism and Developmental Disorders, 44, 31–40. 10.1007/S10803-013-1849-0 [DOI] [PubMed] [Google Scholar]
- Sacker A, Quigley MA, & Kelly YJ (2006). Breastfeeding and developmental delay: Findings from the Millennium Cohort Study. Pediatrics, 118, e682–e689. 10.1542/peds.2005-3141 [DOI] [PubMed] [Google Scholar]
- Sanchez CE, Barry C, Sabhlok A, Russell K, Majors A, Kollins SH, & Fuemmeler BF (2018). Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obesity Reviews, 19(4), 464–484. 10.llll/obr.12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Say GN„ Karabekiroglu K„ Babadagi Z„ & Yiice M. (2016). Maternal stress and perinatal features in autism and attention deficit/hyperactivity disorder. Pediatrics International, 58,265–269. 10.l11l/ped.12822 [DOI] [PubMed] [Google Scholar]
- Schoenfelder EN, & Kollins SH (2016). Topical Review: ADHD and Health-Risk Behaviors: Toward Prevention and Health Promotion. Journal of Pediatric Psychology, 41, 735–740. 10.1093/jpepsy/jsvl62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciberras E, Mulraney M, Silva D, & Coghill D. (2017). Prenatal Risk Factors and the Etiology of ADHD-Review of Existing Evidence. Current Psychiatry Reports, 19,1. 10.1007/s11920-017-0753-2 [DOI] [PubMed] [Google Scholar]
- Sciberras E, Ukoumunne OC, & Efron D. (2011). Predictors of parent-reported attention-deficit/hyperactivity disorder in children aged 6–7 years: A national longitudinal study. Journal of Abnormal Child Psychology, 39, 1025–1034. 10.1007/S10802-011-9504-8 [DOI] [PubMed] [Google Scholar]
- Shih P, Huang CC, Pan SC, Chiang TL, & Guo YL (2020). Hyperactivity disorder in children related to traffic-based air pollution during pregnancy. Environmental Research, 188, 109588. 10.1016/j.envres.2020.109588 [DOI] [PubMed] [Google Scholar]
- Silva D, Colvin L, Hagemann E, & Bower C. (2014). Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics, 133, el4–22. 10.1542/peds.2013-1434 [DOI] [PubMed] [Google Scholar]
- So M, Dziuban EJ, Pedati C, Holbrook JR, Claussen AH, O’Masta B, Maher B, Cerles A, Mahmooth Z, MacMillan L, Kaminski JW, & Rush M. (under review in this issue). Attention-deficit/hyperactivity disorder and childhood physical health: a systematic review and meta-analysis. Prevention Science. [DOI] [PMC free article] [PubMed]
- Smith TF, Schmidt-Kastner R, McGeary JE, Kaczorowski JA, & Knopik VS (2016). Pre- and perinatal ischemia-hypoxia, the ischemia-hypoxia response pathway, and ADHD risk. Behavior Genetics, 46, 467–477. 10.1007/S10519-016-9784-4 [DOI] [PubMed] [Google Scholar]
- St. Sauver JL, Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, & Jacobsen SJ (2004). Early life risk factors for attention-deficit/hyperactivity disorder: A population-based cohort study. Mayo Clinic Proceedings, 79, 1124–1131. 10.4065/79.9.1124 [DOI] [PubMed] [Google Scholar]
- Stadler DD, Musser ED, Holton KF, Shannon J, & Nigg JT (2016). Recalled initiation and duration of maternal breastfeeding among children with and without ADHD in a well characterized case-control sample. Journal of Abnormal Child Psychology, 44, 347–355. 10.1007/sl0802-015-9987-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JC, Everson PM, Williams DC, Hipskind G, Grimes M, & Mahoney ER (2007). Attention deficit/hyperactivity disorder (ADHD) symptoms and digit ratios in a college sample. American Journal of Human Biology, 19,41–50. 10.1002/ajhb.20571 [DOI] [PubMed] [Google Scholar]
- Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Gissler M, & Sourander A. (2018). Lower Apgar scores and Caesarean sections are related to attention-deficit/hyperactivity disorder. Acta Paediatrica, 107,1750–1758. 10.llll/apa.14349 [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Nousen EK, & Chamlou KA (2014). Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiology and Behavior, 123, 236–242. 10.1016/j.physbeh.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MC„ Msall ME„ & Miller RJ (2012). 17-year outcome of preterm infants with diverse neonatal morbidities: Part 1-Impact on physical, neurological, and psychological health status. Journal for Specialists in Pediatric Nursing: JSPN, 17, 226–241. 10.llll/j.1744-6155.2012.00337.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, & Song F. (2000). Methods for meta-analysis in medical research. New York: J. Wiley Chichester, 10.1002/sim.1565 [DOI] [Google Scholar]
- Tervo T, Michelsson K, Launes J, & Hokkanen L. (2017). A prospective 30-year follow-up of ADHD associated with perinatal risks. Journal of Attention Disorders, 21, 799–810. 10.1177/1087054714548036 [DOI] [PubMed] [Google Scholar]
- Tseng PT, Yen CF, Chen YW, Stubbs B, Carvalho AF, Whiteley P, Chu CS, Li DJ, Chen TY„ Yang WC„ Tang CH„ Liang HY„ Yang WC„ Wu CK„ & Lin PY (2019). Maternal breastfeeding and attention-deficit/hyperactivity disorder in children: A meta-analysis. European Child & Adolescent Psychiatry, 28,19–30. 10.1007/s00787-018-1182-4 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau. (2013). Child Health USA 2013. Rockville, Maryland, https://mchb.hrsa.gov/sites/default/files/mchb/Data/Chartbooks/childhealth2013.pdf. Accessed 6 May 2021. [Google Scholar]
- Vanderbilt D, & Gleason MM (2010). Mental health concerns of the premature infant through the lifespan. Child and Adolescent Psychiatric Clinics of North America, 19(2), 211–228. 10.1016/j.chc.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Wagner AI, Schmidt NL, Lemery-Chalfant K, Leavitt LA, & Goldsmith HH (2009). The limited effects of obstetrical and neonatal complications on conduct and attention-deficit hyperactivity disorder symptoms in middle childhood. Journal of Developmental and Behavioral Pediatrics: JDBP, 30,217–225. 10.1097/DBP.0b013e3181a7ee98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfish M, Neuman A, & Wlody D. (2009). Maternal haemorrhage. British Journal of Anaesthesia, 103, i47–56. 10.1093/bja/aep303 [DOI] [PubMed] [Google Scholar]
- Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, & Hertz-Picciotto I. (2015). Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatrics, 169,154–162. 10.1001/jamapediatrics.2014.2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y„ Hu D„ Chen W„ Xue H„ & Du Y. (2019). Prenatal tobacco exposure modulated the association of genetic variants with diagnosed ADHD and its symptom domain in children: A community based case-control study. Scientific Reports, 9,4274. 10.1038/s41598-019-40850-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs K„ Elmore AL„ Nigg JT„ & Nikolas MA (2016). Pre- and perinatal risk for attention-deficit hyperactivity disorder: Does neuropsychological weakness explain the link? Journal of Abnormal Child Psychology, 44,1473–1485. 10.1007/s10802-016-0142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Hao JH„ Tao RX, Huang K„ Jiang XM„ Zhu YD„ & Tao FB (2015). Sex-specific and time-dependent effects of prenatal stress on the early behavioral symptoms of ADHD: A longitudinal study in China. European Child & Adolescent Psychiatry, 24,1139–1147. 10.1007/s00787-015-0701-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.