Abstract
Following excision of colorectal tumors, metastatic disease is prevalent, primarily occurs in the liver, and is highly predictive of poor prognosis. The perioperative period is now recognized as critical in determining the incidence of postoperative metastases and long-term cancer outcomes. Thus, various perioperative prophylactic interventions are currently studied during this time frame. However, immune stimulation during the perioperative period has rarely been attempted due to specific contraindications to surgery and various adverse effects. Here, to prevent liver metastases, we perioperatively employed a TLR-9 agonist, CpG-C, which exhibits minimal pyrogenic and other adverse effects in patients. We found that marginating-hepatic (MH) cells in BALB/c mice contained high percentage of NK cells, but exhibited negligible NK cytotoxicity, as previously reported in humans. However, a single CpG-C administration (25-100 µg/mouse) doubled MH-NK cell numbers, increased NK cell activation and maturation markers (NKp46, CD11b), decreased the inhibitory NKG2A ligand, and dramatically increased MH-NK-cell cytotoxicity against the syngeneic CT26 colon cancer line. Moreover, in operated mice, this innocuous intervention also markedly improved resistance to CT26 and MC38 hepatic metastases in BALB/c and C57BL/6 mice, respectively. Beneficial effects of CpG-C were mediated through activation of MH-NK cells, as indicated by an in vivo NK depletion study. Last, CpG-C protected against surgery-induced suppression of MH-NK cytotoxicity and improved their activation indices. Thus, we suggest that systemic perioperative CpG-C treatment should be considered and studied as a novel therapeutic approach to improve long-term cancer outcomes in colorectal cancer patients.
Keywords: CpG-C, Immunotherapy, Perioperative period, Marginating hepatic NK cells, Colorectal cancer, Liver metastasis
Introduction
Colorectal carcinoma (CRC) is a prevalent and fatal malignancy, accounting for 54,611 deaths in the USA alone in 2018 [1]. Metastatic disease of colorectal cancer primarily occurs in the liver, is highly predictive of poor prognosis, and constitutes the major cause of colorectal cancer mortality [2]. The liver filters the venous drainage of the majority of intra-abdominal viscera, as well as approximately 30% of other venous blood returning to the heart. Thus, liver resistance to circulating CRC cells and metastasis seems critical for long-term outcomes of CRC. Initial detection of a primary CRC usually occurs when the metastatic process is already ongoing and is manifested in detectable macro-metastases, undetectable micrometastases, or single tumor cells in the circulation or lymphatic system [3].
The perioperative period (days before and after surgery) is now recognized as a critical time frame, impacting the risk for metastatic disease non-proportionally to its short duration [4]. Specifically, by removing the primary tumor, surgery presents a window of opportunity for the immune system to control or eliminate the remaining minimal residual disease (MRD) through (i) terminating the common immunosuppressive effects of the primary tumor, and (ii) eliminating the major source of metastasizing cancer cells [3]. MRD, and especially circulating tumor cells, is more susceptible to immune control than the primary tumor and can thus be eliminated before extravasating and establishing metastatic foci. On the other hand, the surgical procedure itself promotes the initiation of new metastasis and stimulates the progression of preexisting MRD, through numerous synergistic mechanisms, including (i) increasing tumor cell shedding into the circulation [5], (ii) inducing immune suppression [6], and (iii) reducing the levels of angiostatic agents while increasing the levels of growth factors and pro-angiogenic factors [7]. Therefore, the perioperative period has a multifaceted and critical impact on long-term cancer outcome. Accordingly, exploiting this underutilized time frame for anti-metastatic interventions may reduce recurrence rates, as suggested by others and us [4, 8].
An important and relatively unattended immune mechanism preventing metastases are specialized marginating immunocytes–leukocytes that adhere and reside within the capillaries of various organs, specifically the liver and the lungs [9, 10]. These cells are strategically located to physically interact with all circulating aberrant cells, which are forced to slow their progression through the capillaries, deforming and rolling along the surface of these resident leukocytes [11]. This process enables recognition and elimination of malignant cells by the marginating immunocytes. Indeed, marginating-pulmonary (MP)-NK cells and marginating-hepatic (MH)-NK cells (also known as Pit cells) were suggested to play a crucial role in preventing blood-borne metastases [9]. Unfortunately, hepatic leukocytes, including MH-NK cells, are known to be in a state of hypo-responsiveness or tolerance, at least in mice and humans, thus potentially rendering the liver susceptible to metastatic colonization by circulating tumor cells [12].
Considering the above, immune stimulation of marginating NK cells during the perioperative period has a potential to markedly reduce long-term recurrence rates. However, perioperative immune stimulation has rarely been attempted, given specific contraindications to surgery by most immune-stimulating agents (e.g., IL-2). Severe systemic adverse responses are induced by most of these agents, including life-threatening pyrogenic and inflammatory effects that are indistinguishable from signs of infections [13].
To overcome these obstacles, we used a fast-acting immune-stimulating agent known to exhibit minimal adverse effects in patients, which can thus be more safely used within the perioperative time frame. Oligodeoxynucleotides (ODN) containing unmethylated CpG dinucleotides (CpG ODN) mimic the immune stimulatory activity of microbial DNA via activation of toll-like receptor (TLR)-9 [14]. CpG-C is a biologically stable subclass of CpG ODN that activates both innate and adaptive immunity, including B cells, NK cells [15], as well as plasmacytoid dendritic cells (pDC) in human and mice [16]. In hepatitis and in cancer patients, CpG-C is administered systemically and intra-tumorally, respectively, with minimal adverse effects [17]. Additionally, studies in rats showed that beyond a certain dose, increasing quantities of CpG-C do not increase its potency or its minimal adverse effects, apparently as the impact of CpG-C is induced through a natural self-balanced immune responses orchestrated through several leukocyte subtypes (e.g. DC, macrophages) [18, 19] that contain specialized TLR-9 receptors.
Here, we evaluated the efficacy of systemic use of CpG-C for preventing the seeding and establishment of experimental liver metastases. We found that a single administration of CpG-C a day prior to surgery doubled the numbers of MH-NK cells, increased their activation markers, and caused a dramatic increase in MH-NK in vitro cytotoxicity against a syngeneic colon cancer cell line (CT26). Most importantly, following in vivo inoculation of CT26 or MC38 cells through the hepatic portal vein, liver metastases developed only in approximately 10% of mice treated with CpG-C, compared to 80% in non-treated animals, an effect that was mediated through activation of MH-NK cells in the CT26 model.
Materials and methods
Animals and counterbalancing
BALB/c male and female mice and C57B1/6j male and female mice were used at the age of 8–12 weeks. (Animals were age-matched between groups within each experiment.) The order of tumor inoculation and other experimental procedures were counterbalanced across all experimental groups.
Tumor cell lines
The CT26 tumor cell line
The CT26 murine colon carcinoma cell line is a chemically induced undifferentiated carcinoma, syngeneic to the BALB/c strain [20]. Cells were grown in monolayer cultures in 37 °C, 100% humidity, 5% CO2, in complete medium (CM) (RPMI 1640, supplemented with 10% heat-inactivated fetal calf serum (FCS), 0.05 mg/ml gentamicin, 2 mM l-glutamine, 1 mM sodium pyruvate, and 0.1 mM non-essential amino acids.
Preparation of CT26 tumor cells for injection
Cells were removed from the culture flask with a trypsin solution (0.25% in PBS), washed once in PBS containing 0.1 mg/ml BSA (335 g for 10 min.), and adjusted to a final concentration of 1 × 105/ml in PBS supplemented with 0.1% BSA for either portal vein or splenic injection in a volume of 100 µl per animal.
The MC38 tumor cell line
The MC38 murine adenocarcinoma was derived from a colon tumor that arose in C57B1/6 mice.
Preparation of MC38 tumor cells for injection
Cells were removed from the culture flask with a trypsin solution (0.02% in PBS), washed once in PBS containing 0.1 mg/ml BSA (335 g for 5 min), and adjusted to a final concentration of 1 × 104/ml or 5 × 104/ml in PBS supplemented with 0.1% BSA for splenic injection in a volume of 100 μl per animal.
Drugs and their administration
CpG ODN
A type C CpG ODN (ODN 2395: 5′-CGTCGTTTTCGGCGCGCGCCG-3′) with a phosphorothioate backbone was used. This ODN has been demonstrated to retain characteristics of both type A and B CpG ODNs by exhibiting potent activation of both innate and adaptive immune responses. In experiments utilizing the CT26 tumor cell line, CpG-C was injected i.p. at doses of 12, 25, 50, and 100 µg/animal, in 0.1 ml of PBS supplemented with 0.1% BSA. In the experiment utilizing the MC38 cell line, CpG-C was injected i.p. at doses of 50 µg/animal, in 0.1 ml of PBS supplemented with 0.1% BSA.
Anti-asialo GM1 Ab for depletion of NK cells in BALB/c mice
BALB/c mice were injected i.p. with 30 µl of anti-asialo GM1 (according to manufacturer instructions, Wako, by Enco, Israel), or with PBS as control. This approach is ineffective in C57B1/6j mice.
Surgical procedure and tumor inoculation
Hepatic portal vein inoculation
Anesthesia was initiated by subjecting mice to 6% isoflurane in air for 20–30 s. Isoflurane anesthesia was maintained at 1.5–2.5%; thereafter, the skin was shaved and rubbed with alcohol pads, and a 1.5-cm midline abdominal incision was conducted. The hepatic portal vein was exposed, and 1 × 105 CT-26 tumor cells in 100 µl PBS were injected using a 31G needle, as previously described in detail in [21].
Intra-splenic inoculation
Mice were anesthetized as described above. The skin was shaved and rubbed with ethanol pads, and a 0.5-cm abdominal incision was made adjacent to the spleen (a left flank incision approximately 2 cm left of the abdominal midline). Tumor cells were injected into the spleen using a 31G needle, which was maintained in the spleen tissue for 2 min. Following injection, a 4/0 (in the CT26 experiments) or 5/0 (in the MC38 experiment) polypropylene monofilament non-absorbable suture was placed across the hilum of the spleen to prevent bleeding, and a splenectomy was then performed. Following the excision of the injected spleen, the peritoneum and skin were sutured with 4/0 non-absorbable filaments (in the CT26 experiments) or glued with 3 M vetbond tissue adhesive (in the MC38 experiment). Animals were allowed to recover in their home cages.
Assessment of metastatic development
Following tumor inoculation via either the portal vein or the spleen injection approaches, animals were monitored daily for general well-being and were euthanized with an overdose of isoflurane on the 21st day in the CT26 experiments and on the 24th day in the MC38 experiment. Livers were then harvested and weighed, and surface-hepatic metastases were counted by two investigators blinded to each animal’s experimental group, as previously described in detail in [21].
Harvesting of marginating-hepatic (MH) leukocytes
Mice are sacrificed with an overdose of isoflurane, and the peritoneal and chest cavities were opened, sparing the rostral aspect of the diaphragm intact. Marginating-hepatic leukocytes were harvested by perfusing the liver with heparinized PBS (30 U/ml), as previously detailed in [22].
Assessment of NK cytotoxicity
The standard 4-h 51Cr release assay was used without any cell enrichment procedures. This technique assesses anti-tumor NK cell cytotoxicity (NKCC) per the sample tested. Previous studies have indicated that the cytotoxicity measured using this technique is ascribed to NK cells, rather than other cell types, as selective depletion of NK cells eliminates all target cell killing. The advantages of this procedure include shorter duration, less interference with the effector cells, and improved representation of the original in vivo cell composition. This procedure is specified in [9].
Flow cytometry
Sample preparation and analysis
A 50 µl aliquot of liver perfusate was added to 50 µl of PBS supplemented with 2% FCS and 0.1% NaN3 (PBS ++) containing a set of conjugated antibodies (see below). Sample preparation is detailed in [23].
Identification of leukocyte subsets and cellular expression markers
FACS analysis was used to quantify different leukocyte subsets in the MH-compartment, as well as their expression level of different surface molecules. Granulocytes and lymphocytes were identified based on forward by side scatter. The following cell populations were identified based on their CD presentation, using two different sets of specific mAbs: Within lymphocytes, NK cells were identified as being PE-conjugated anti-mouse NKp46 (clone 195314, R&D systems) and FITC conjugated CD49b bright cells (clone DX5, biogems, Peprotech Asia). The expression of the adhesion molecule CD11b (clone M1/70, BioLegend) was identified using APC-Alexa Fluor 750-conjugated, and the expression of the inhibitory molecule NKG2A (clone #131411, R&D systems) was identified using APC-conjugated antibody.
Statistical analysis
Depending on experimental design, we conducted one- or two-way factorial analyses of variance (ANOVA) with a predetermined significance level of 0.05, to assess the number of metastases and liver weight, and to assess the number of NK cells, their expression levels, and their surface markers between the different groups. When NK cytotoxicity was assessed, 2 × 5 repeated-measures ANOVA was conducted (repeated E/T ratios). When ANOVA indicated significant group differences, post hoc contrasts were performed (Fisher’s PLSD) based on a priori hypotheses. Data were always assessed to verify normal distribution and group homogeneity of variance and presented as mean ± SEM.
Results
Exp. 1: Immune stimulation with CpG-C markedly increases numbers and cytotoxicity levels of MH-NK cell
Design and procedure
CpG-C (100 µg/mouse, i.p.) or vehicle was injected 24 h prior to sacrificing the mouse and perfusing the liver (n = 9 & 4, respectively). MH-leukocytes were collected, washed twice, and concentrated to a final volume of 800 µl (see Materials and methods). Each sample of MH-leukocytes was used for the assessments of the number of MH-NK cells, based on lymphocytes expressing CD49b, and for the assessment of NK cytotoxicity against the syngeneic CT26 target cells (see methods).
Result
NK cell cytotoxicity
Repeated-measures ANOVA (CpG-C treatment and repeated E/T ratios) indicated that CpG-C administration elevated NK cytotoxicity against CT26 ((F(1,11) = 8.815, p = 0.0128) (Fig. 1a). This elevation reflects up to sevenfold increase in cytotoxicity per samples tested, when comparing absolute levels of killing (e.g., 28% vs. 4% cytotoxicity). However, the horizontal shift in the cytotoxicity curves caused by CpG-C is more than 4 E/T ratios, reflecting at lease 16-fold (24) increase in cytotoxicity per sample taken from CpG-treated animals.
Fig. 1.
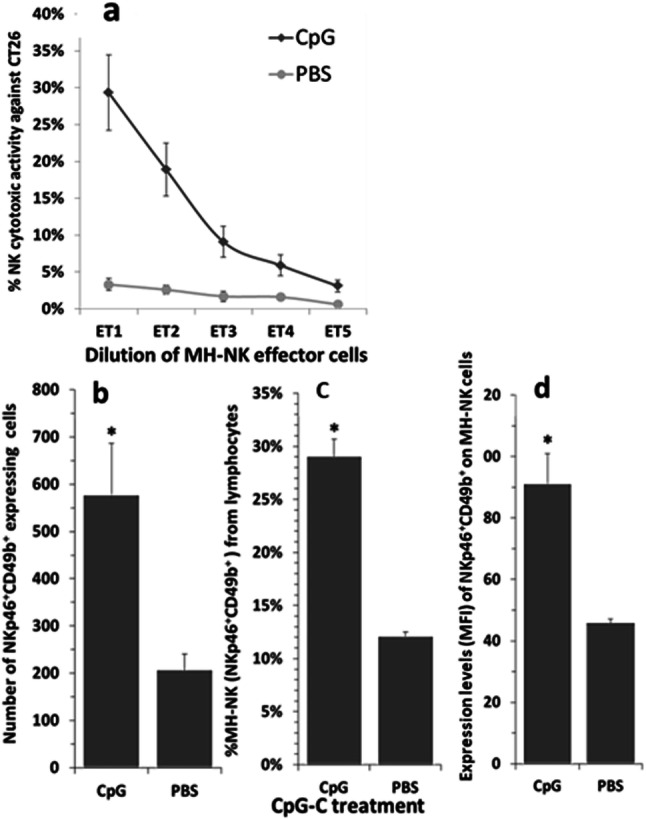
Effects of CpG-C treatment on numbers and cytotoxicity of MH-NK cells and on CD49 expression levels (MFI). Mice were challenged with CpG-C or PBS and 24 h later were sacrificed, their livers were perfused to collect MH-NK cells, and NK number and activity against syngeneic CT26 target cells were assessed. CpG-C administration significantly elevated NK cytotoxicity (a). ET represents the effector cell-to-target cell ratio achieved by twofold serial dilution of MH-leukocytes. CpG-C treatment also significantly elevated the total number of MH-NK cells (CD49+ lymphocytes) (b), and their percentage within the lymphocyte population (c). Last, expression levels of CD49 on NK cells were also elevated by CpG-C (d). Data are expressed as mean + SEM. * indicates a significant difference from PBS control levels
Total MH-NK cells number and % of NK cells from lymphocytes
One-way ANOVA indicated that CpG-C treatment significantly elevated the total number of MH-NK cells (F(1,11) = 9.188, p = 0.0114) (Fig. 1b) and their percentage within the lymphocyte population (F(1,11) = 85.335, p < 0.0001) (Fig. 1c), reaching approximately 2.5-fold increase. Expression level of CD49b was also elevated by CpG-C (F(1,11) = 18.102, p = 0.0014) (Fig. 1d).
Overall, the 2.5-fold increase in the numbers of MH-NK cells can explain only a portion of the sevenfold increase in cytotoxicity of the entire MH-NK cells population. Thus, an increased cytotoxicity per MH-NK cell also occurs and seems to explain the greater portion of the total increase in liver NK cytotoxicity (7–16 folds).
Exp. 2: CpG-C administration markedly decreases the number of surface-hepatic metastases and liver weight in a dose-dependent manner
CT26 experiments
Design and procedure
To evaluate the potential biological significance of CpG-C immune stimulation in reducing liver metastases, mice were injected with CpG-C or with vehicle. In the first study (n = 17 and 11, respectively), CpG-C was injected i.p. twice in a dose of 100 µg/mouse, 24 h prior to tumor cell inoculation, and once again 24 h following inoculation. In the second study, CpG-C was administered once, 24 h prior to tumor inoculation, using lower doses of 0 (vehicle control) 12, 25, and 50 µg/mouse (n = 12, 12, 12, and 10, respectively). Tumor cells were injected via the portal vein. All mice were sacrificed 21 days following tumor inoculation, their livers were removed and weighed, and surface-hepatic metastases were counted by two researchers blinded to the experimental group assignment.
Results
In the first study, CpG-C treatment significantly reduced both the number of surface-hepatic metastases and liver weight, as indicated by one-way ANOVAs (F(1,26) = 33.321, p < 0.01 & F(1,26) = 23.481, p < 0.01, respectively) (Fig. 2a + B). The second study indicated dose-dependent beneficial effects of a single CpG-C administration. While the dose of 12 µg did not significantly reduce the number of metastases, the doses of 25 and 50 µg significantly reduced surface-hepatic metastases (F(3,42) = 4.444, p < 0.05) and liver weight (F(3,43) = 4.475, p < 0.05) (PLSD, p < 0.05 for these pair-wise comparisons) (Fig. 3a + b).
Fig. 2.
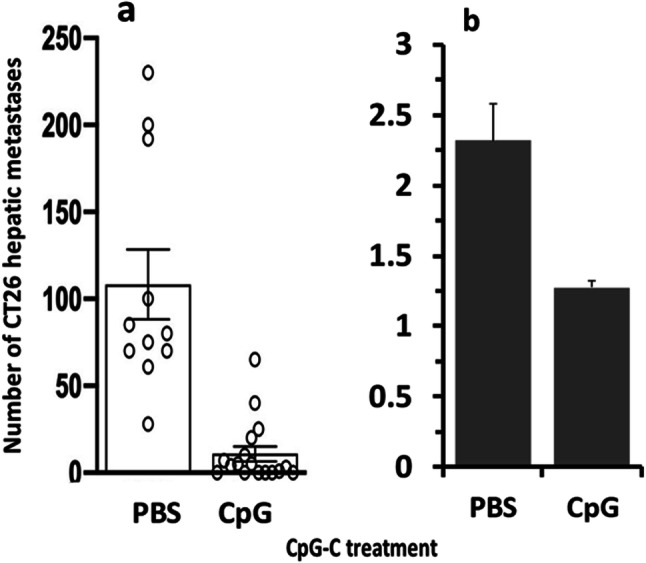
CpG-C hinders the development of CT26 surface-hepatic metastases and reduces liver weight. CpG-C (100 µg/mouse) or PBS (control) were injected twice, 24 h prior and 24 h following 20,000 CT26 tumor cell inoculation. CpG-C treatment significantly reduced number of metastases (a), and liver weight (b). The average weight of livers without metastases is approximately 1 g. Data are expressed as mean + SEM. * indicates a significant difference from PBS control levels
Fig. 3.
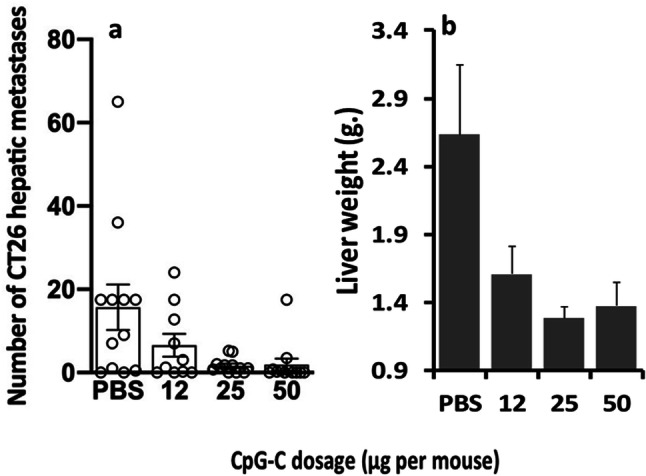
CpG-C administration markedly decreased the number of CT26 surface-hepatic metastases and liver weight in a dose-dependent manner. CpG-C was administered once, 24 h prior to 10,000 CT26 tumor inoculation, in doses of 0 (PBS control) 12, 25, and 50 µg/mouse. Dose-dependent beneficial effects of these single CpG-C administration were evident. While the dose of 12 µg did not significantly reduce number of metastases, the doses of 25 and 50 µg significantly reduced surface-hepatic metastases (p < 0.05) (a) and liver weight (b). Data are expressed as mean + SEM. * indicates a significant difference from the PBS group
MC38 experiment
To further evaluate and ascertain the biological significance of systemic CpG-C immune stimulation, we used an additional syngeneic model of colorectal cancer in a different strain of mice and assessed the efficacy of CpG-C treatment in inhibiting the establishment of experimental MC38 hepatic metastases in C57B1/6 male and female mice.
Mice were injected once with CpG-C (n = 12) or vehicle (PBS) (n = 11) and were further subdivided into two groups—inoculated with 10,000 (n = 12) or 50,000 (n = 11) MC38 cells via intra-splenic inoculation. CpG-C was injected i.p. at a dose of 50 µg/mouse, 24 h prior to tumor inoculation. All mice were sacrificed 24 days following the inoculation of tumor cells, their livers were removed, and surface-hepatic metastases were counted. CpG-C administration significantly reduced surface-hepatic metastases (Fig. 4a) (p < 0.05) and had a marginally significant effect on liver weight (Fig. 4b) (p = 0.078). No sex differences were observed.
Fig. 4.
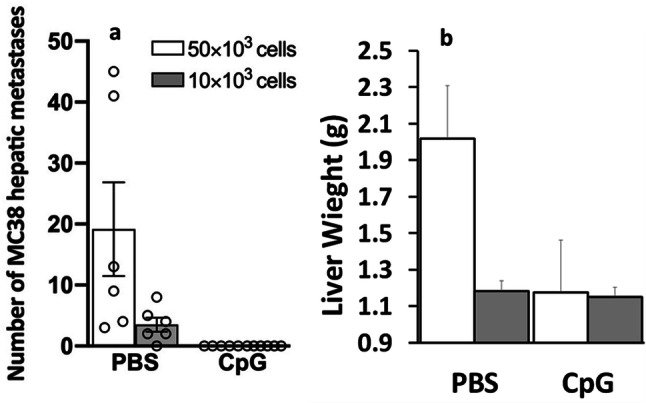
CpG-C administration markedly decreases the number of MC38 surface-hepatic metastases. A single 50 µg/mouse CpG-C treatment was given 24 h prior to 10,000 or 50,000 MC38 tumor cell inoculation. CpG-C administration significantly reduced (a) surface-hepatic metastases and (b) had a marginally significant effect on liver weight (p = 0.078). Data are expressed as mean + SEM. * indicates a significant difference from the no-CpG group
Exp. 3: NK cells depletion significantly elevated the number of surface-hepatic metastases and liver weight and prevented the effects of CpG-C
Design and procedure
To evaluate the role of NK cells in controlling hepatic metastases of the CT26 tumor cell line, and the potential involvement of NK cells in mediating the beneficial effects of CpG-C immune stimulation, a total of 37 female mice were injected with either anti-asialo GM1 (n = 20) or with vehicle (n = 17) and were further subdivided to receive either CpG-C (n = 17) or vehicle (n = 20). Anti-asialo GM1 and CpG-C were injected i.p. at doses of 30 µl (in a total volume of 100 µl PBS), and 100 µg/mouse, respectively. 24 h later, 2 × 104 CT26 tumor cells were injected per mouse using the intra-splenic inoculation approach. All mice were sacrificed 21 days following tumor inoculation, their livers were removed and weighed, and surface-hepatic metastases were counted by two researchers blinded to the experimental group assignment.
Results
NK depletion increased the number of hepatic metastases by approximately sevenfold (F(1,33) = 22.115, p < 0.0001) (Fig. 5), and a similar effect was evident in liver weight (F(1,33) = 14.958, p = 0.0005), as indicated by a significant main effect in the ANOVA (data not shown). CpG-C alone (in animals in which NK cells were not depleted) reduced the number of metastases from an average of 10 to 0 in all mice except for one animal that exhibit one metastasis. Importantly, CpG-C treatment in NK-depleted mice did not reduce the number of metastases (Fig. 5). Overall, these findings indicate that NK cells are pivotal in controlling CT26 hepatic metastasis, and that CpG-C acts through NK activation to reduce the number of hepatic metastases.
Fig. 5.
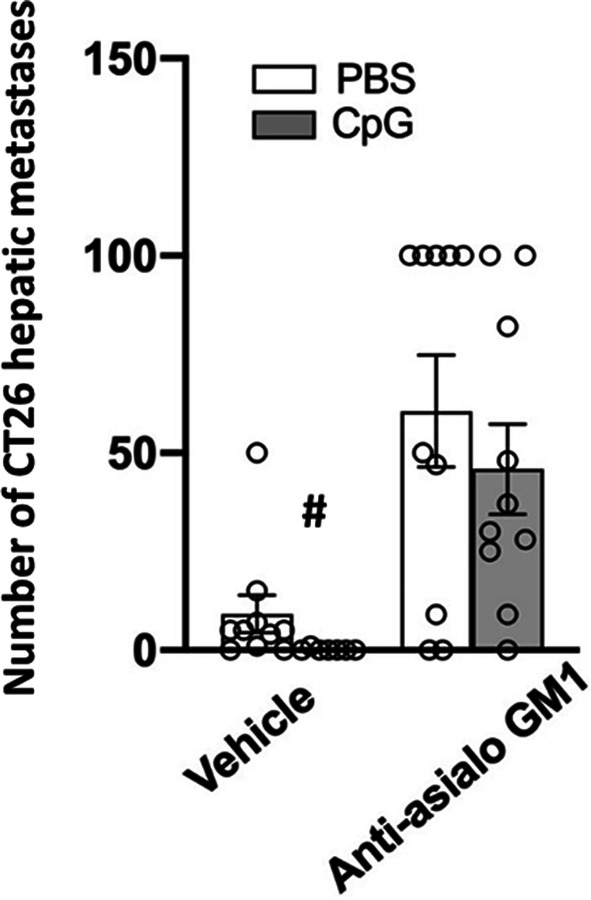
NK cells depletion significantly elevated the number of CT26 surface-hepatic metastases and liver weight, and CpG-C significantly reduced number of metastases in intact mice. A day before tumor inoculation (20,000 cells), female mice were administered with PBS or CpG-C (100 µg/mouse), and were further subdivided to receive PBS or anti-asialo GM1 for NK depletion. NK depletion significantly increased the number of hepatic metastases. In mice with intact NK cells, CpG-C significantly reduced the number of metastases from an average of 10 to 0.1. In NK-depleted mice, CpG-C treatment did not cause a significant reduction in the number of metastases. Data are expressed as mean + SEM. # indicates a significant difference from the PBS
Exp. 4: No effects of surgery on NK cytotoxicity is evident in CpG-C-treated animals, and protective effects of CpG-C against the deleterious effects of surgery on related NK cell markers
Design and procedure
Mice were administered with CpG-C (100 µg/mouse, i.p.) (n = 19) or vehicle (n = 6) and 24 h later were further subdivided to undergo either laparotomy (n = 12) or to serve as home cage controls (n = 13). Ten hours following surgery, all mice were sacrificed, their livers were perfused, and MH-leukocytes were collected, washed twice, and brought to a final volume of 800 µl (see Materials and methods). Each sample of MH-leukocytes was used for the assessments of NK cytotoxicity against the syngeneic CT26 target cells. These same samples were also used for the different FACS analyses conducted on NK cell surface markers. (Each sample was used to quantify several, but not all FACS markers.)
Results
NK cell cytotoxicity
Two-way repeated-measures ANOVA (CpG-C treatment, surgery, and repeated E/T ratios) indicated a significant and marked increase in NK cytotoxicity by CpG-C administration (main effect), against CT26 (Fig. 6a) (F(1,21) = 6.540, p = 0.0183) target cells, but no effects for surgery. Given the very low levels of NKCC in animals not treated with CpG-C (floor effect), these findings can only suggest no deleterious effects of surgery in CpG-C-treated animals, which showed marked levels of NKCC.
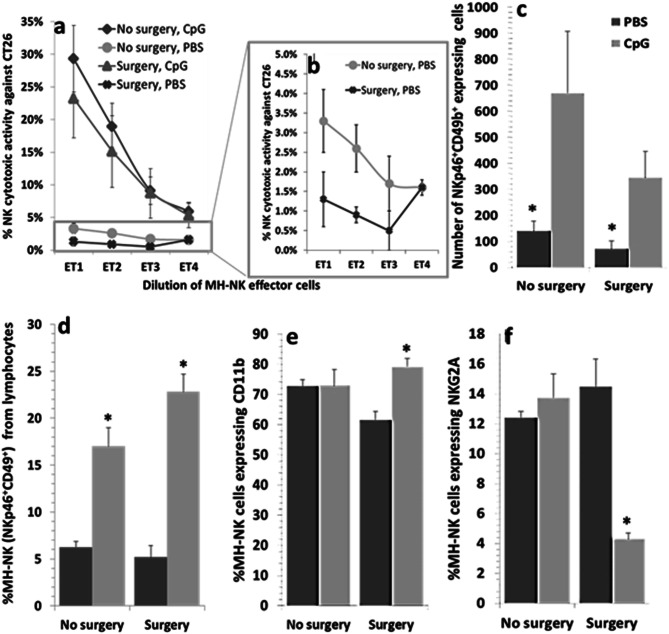
Fig. 6.
Protective effects of CpG-C against the deleterious effects of surgery on NK cytotoxicity, and on cytotoxicity-related NK cell markers. Mice were administered with CpG-C (100 µg/mouse, i.p.) or PBS (control) and 24 h later were further subdivided to undergo laparotomy or to serve as home cage controls. CpG-C administration resulted in a significant and marked increase in NK cytotoxicity against CT26 target cells (a), and no effects for surgery on cytotoxicity were evident in these CpG-C treated mice, despite a reduction in the number of MH-NK cells induced by surgery (NKp46 + lymphocytes) (c). In animals not treated with CpG-C, cytotoxicity levels were extremely low, probably indicating a floor effect, hindering our ability to infer about potential effects of surgery (b). The protective effects of CpG-C against the influence of surgery are also evident with respect to the percentage of MH-NK cells (NKp46 +) within the lymphocyte population (d), and the percentage of MH-NK cells expressing the murine maturation CD11b index (e) or the inhibitory NKG2A marker (f). In these three outcomes (d, e, f), CpG-C transverse the deleterious effects of surgery evident in non-CpG-C-treated animals, yielding a significant interaction between CpG-C treatment and surgery in a manner that is associated with increased NK activity
Number, percent of MH-NK from lymphocytes, and percent of MH-NK expressing stimulatory and inhibitory markers
In general, CpG-C treatment improved all indices studied herein that underlie or associate with improved NK activity. Specifically, CpG-C treatment leads to (i) an increase in NK cell number and % NK cells from lymphocytes, (ii) an increased expression levels or percent of MH-NK cells expressing NKp46 and CD11B, and (iii) reduced % of the NK-inhibitory marker NKG2A. Surgery either worsened these indices or had no effect in naïve animals, but improved these indices in CpG-C-treated animals, attesting to the protective effects of CpG-C against the deleterious effects of surgery.
Specifically, NKp46 was used to identify NK cells within the lymphocyte population. Two-way ANOVA (CpG-C treatment and surgery) indicated that CpG-C treatment significantly elevated the total number of MH-NK cells (F(1,4) = 9.488, p = 0.0369) (Fig. 6c), and their percentage within the lymphocyte population (F(1,4) = 86.546, p = 0.0007) (Fig. 6d), reaching approximately a threefold increase. Expression levels of NKp46 were elevated by CpG-C (F(1,11) = 20.593, p = 0.0105), without an effect for surgery (data not shown).
Importantly, surgery reduced the percent of NK cells from lymphocytes population in control animals, but increased it in CpG-C-treated animals, reaching a marginally significant interaction (F(1,4) = 5.115, p = 0.0865) (Fig. 6d).
Percent of CD11b+ within the NK cell population was increased by CpG-C treatment (F(1,4) = 10.326, p = 0.0058), and a marginally significant interaction between CpG-C treatment and surgery was evident (F(1,4) = 6.379, p = 0.0650), where surgery increased percent of CD11B+ within CpG-C-treated animals, but not in control animals (Fig. 6e).
A significant interaction between CpG-C treatment and surgery was evident with respect to percent of NKG2A+ cells within NK cells (F(1,4) = 21.689, p = 0.0096). Specifically, surgery reduced this inhibitory index in CpG-C-treated animals, but had no effect in non-treated animals (Fig. 6f).
Discussion
The microcirculation in the liver is convoluted, and blood flow is slow as a result of (i) the anastomotic display of networking sinusoidal capillaries within hepatic lobules, and (ii) the control over blood flow by intra-sinusoidal macrophages (Kupffer cells) and perisinusoidal stellate cells [24]. These structural and hemodynamic characteristics of the liver foster physical interactions between marginal resident leukocytes and circulating tumor cells, which are retained by the liver while passing through the sinusoidal capillaries. Thus, we first studied the efficacy of a single systemic administration of CpG-C in activating MH-NK cells, which are strategically located to identify and kill tumor cells in the liver. To this end, we employed a new method for harvesting leukocytes from the liver sinusoids, through forced perfusion [22], instead of the common approaches that include initial grinding of the liver and isolation of lymphocytes, which may lead to the secretion of multiple immune modulating factors and the delayed assessment of NK activity in an artificial composition of leukocytes [25]. We found that a single administration of 100 μg of CpG-C, a day prior to MH cell harvesting, led to (i) doubling of the number of MH-NK cell per liver, and doubling of their proportion within the MH-lymphocyte population, (ii) increased expression levels of the CD49 maturation and activation [26] marker on MH-NK cells, and (iii) a dramatic 10–20-fold increase in total MH-NK cell cytotoxicity against syngeneic CT26 tumor cells. Overall, the 2.0–2.5-fold increase in the numbers/percentage of MH-NK cells can explain only a portion of the greater (10-20 fold) increase in cytotoxicity of the MH-NK cells population. Thus, an increased cytotoxicity per MH-NK cell also occurred, apparently underlying the majority of the increase in liver MH-NK cytotoxic capacity (10–20-fold).
The increase in MH-NK cytotoxicity per cell caused by CpG-C may be mediated through various known mechanisms. TLR-9 has been found to be the primary receptor for CpG ODNs [27]. The complex immunologic responses to CpG ODNs by various subsets of immunocytes involve both direct and indirect effects [28], resulting in the activation of NK cells, T cells, B cells, monocytes, macrophages, and dendritic cells [14, 29]. Specifically, the activation of TLR-9 by CpG-C results in a powerful TH1 response, with both plasmcytoid dendritic cells (pDCs) and B cells contributing to this effect [30, 31]. For instance, CpG-A and CpG-C were shown to cause secretion of the pro-inflammatory cytokine IL-12 by pDC, and consequently to the activation of NK cells [32]. Additionally, CpG-C induces interferon-alpha (IFN-alpha) secretion by pDC [18], which in turn induces the release of co-stimulatory factors such as CD80, TNF-related apoptosis-inducing ligand (TRAIL), and chemokine receptor 7 (CCR7) in monocytes [33].
An important aspect of the current finding is the low functional capacity of MH-NK cells cytotoxicity (NKCC) in naïve mice (not treated with CpG-C) against the syngeneic CT26 tumor line (~ 3%). The liver is continuously exposed to foreign antigens derived from food and commensal flora [34], and some assert that such a condition necessitates local immune suppression to avoid ongoing inflammation. Indeed, mechanisms underlying such liver tolerance include activation of regulatory T cells [35], the local production of immunosuppressive cytokines such as IL-10 [36], and eradication of activated T cells [37]. Given our current findings and other reports [36], an additional aspect of liver tolerance is the hypo-responsive state of MH-NK cells. NK cells represent a large proportion of the lymphocyte population in the liver and may also be involved in maintaining liver tolerance through their interactions with a variety of cell types and their pivotal role in secreting pro- and anti-inflammatory cytokines [12]. In a recent study, NK cells co-cultured with hepatocytes were shown to alter the ability of dendritic cells (DC) to prime CD4+ T cells, resulting in a regulatory T cell phenotype and function [38]. Importantly, DC induction of this T cell regulatory phenotype was dependent on the engagement of the NK cell inhibitory receptor NKG2A with hepatocytes, directly implicating MH-NK cells in this suppression. In the current study, CpG-C reduced levels of NKG2A expression on MH-NK cells in the context of surgery, potentially regulating hepatic immune tolerance through this mechanism. Overall, and as evident herein, CpG-C treatment may be an efficient approach to alleviating liver tolerance, activating the MH-NK cell system, and potentially protecting the liver against tumor metastases.
To evaluate the in vivo role of NK cells in controlling hepatic CT26 metastases, and to assess their involvement in mediating the beneficial effects of CpG-C, we depleted NK cells in BALB/c mice, employing anti-asialo GM1. NK depletion significantly increased the number of hepatic metastases by approximately sevenfold (and significantly elevated liver weight), suggesting a role of NK cells in controlling CT26 liver metastasis. CpG-C alone reduced the number of metastases from an average of ~ 9 to 0 in all mice (except one animal that exhibits one metastasis), again indicating the efficacy of CpG-C in controlling liver metastasis. In contrast, within NK-depleted animals, CpG-C treatment had no beneficial effects on the number of metastases. Overall, these findings indicate that NK cells are pivotal in controlling CT26 hepatic metastasis, and that NK cells are key in mediating the beneficial effects of CpG-C against CT26 hepatic metastases. It is worthy to note that anti-asialo GM1 (routinely used for NK depletion) does not exclusively affect NK cells, and that basophiles and other leukocyte subtypes are also affected [39]. Additionally, anti-asialo GM1 was reported not to deplete tissue-resident NK49a + cells [40]. However, the findings that MH-NK cytotoxicity against the same CT26 line is markedly increased within a day of CpG-C treatment, and that anti-asialo GM1 markedly increase numbers of CT26 hepatic metastases, support our assertion that MH-NK cells mediate the effects of CpG-C in this tumor model.
In the clinical setting, surgery is a necessary and often the first line of treatment against CRC. Unfortunately, the surgical procedure and the perioperative period are now believed to also induce pro-metastatic processes, making this short time frame a critical period in determining long-term cancer outcomes. Specifically, surgery is known to suppress NK cell cytotoxicity, and through this suppression to promote metastasis [41, 42]. Therefore, as our future aim is to test CpG-C in the clinical perioperative setting, we also tested the efficacy of CpG-C in animals subjected to surgery. CpG-C significantly increased the numbers of MH-NK cells in both operated and non-operated animals, and surgery decreased these numbers in both CpG-C and vehicle-treated mice. A similar decrease by surgery was previously reported in the numbers of marginating NK cells in the lungs of rats [9, 23] and mice [41]. However, in addition to these simple main effects of CpG-C and of surgery on the numbers of MH-NK cells, surgery induced deleterious effects in non-treated animals, but had no such impact in CpG-treated animals. Specifically, surgery suppressed MH-NK cytotoxicity in control animals but not in CpG-C-treated animals, which showed markedly higher NKCC. Additionally, CpG-C increased the percentage of MH-NK cells within the MH-lymphocyte population more profoundly in the context of surgery. Lastly, surgery reduced the percent of CD11b+ within the MH-NK cell population in naive animals, but increased it in CpG-C-treated animals; and surgery had no effect on percent of NKG2A+ cells within MH-NK cells, but markedly reduced the percent of MH-NK cells that express this inhibitory receptor in CpG-C treated mice.
Overall, despite the fact that surgery significantly reduced MH-NKCC in non-treated animals and reduced by half the number of MH-NK cells in CpG-C treated animal, it did not decrease MH-NKCC in CpG-C-treated animals. This is likely due to increase in both the percent of MH-NK cells that express CD11b and reduction in those that express the inhibitory NKG2A. Integrin CD11b is used in mice as a marker for NK cell maturation, indicating their capacity to produce IFNγ and exhibit cytotoxicity [12, 43]. NKG2A is a pivotal inhibitory receptor expressed by NK cells, and its high expression in mice not treated with CpG-C, both in the laparotomy and in the control groups, coincided with the hypo-responsiveness/tolerance observed in MH-NK cells [36]. However, in mice undergoing surgery, the CpG-C pre-treatment appears to overcome this hypo-responsiveness, harnessing surgery to decrease the inhibitory NKG2A expression in MH-NK cells, concurrently with maintaining high MH-NKCC. Together, these findings indicate that CpG-C treatment not only potentiates the MH-NK cell system, but also protects it from the immune-suppressive effects of surgery, apparently by converting MH-NK cells into a mode where surgical stress positively affects their CD11b and NKG2A ligands, rather than negatively affecting them in naïve mice.
In summary, we show that a single CpG-C treatment in mice converts MH-NK cells from being in a hypo-responsive state to cells with potent in vitro cytotoxic capacity against syngeneic colon cancer cells (CT26) and markedly improves in vivo resistance to colonization of the liver by two colorectal syngeneic tumor lines. Additionally, the same CpG-C treatment, which did not show adverse effects in this and previous studies [44], and is being used in clinical trial [45], also protected MH-NK cell from suppression by surgery, as evident by their cytotoxicity levels and molecular markers of NK cell functions. The different effects of CpG-C on the numbers and on the activity of NK cells in the marginating-hepatic immune compartment warrant further characterization of its effects with respect to basic mechanisms and clinical ramifications. Our findings point to CpG-C treatment as advantageous in abrogating the effects of surgery with no adverse side effects; hence, this new perioperative intervention should be tested in operated colorectal patients to improve long-term cancer outcomes during the critical and unexploited perioperative time frame.
Abbreviations
- CCR7
Chemokine receptor 7
- CpG-C ODN
Oligodeoxynucleotides (ODN) containing unmethylated CpG dinucleotides (CpG ODN)
- CRC
Colorectal cancer/carcinoma
- CT26
Colon tumor cell line
- IFN
Interferon
- MC38
Mouse colon cell line
- MH-NK cell
Marginating-hepatic NK cell
- MP-NK cell
Marginating pulmonary NK cell
- MRD
Minimal residual disease
- NKCC
NK cells cytotoxicity
- pDC
plasmacytoid dendritic cells
- TLR9
Toll-like receptor 9
- TRAIL
TNF-related apoptosis-inducing ligand
Author contributions
LS is the lead researcher of the studies presented in this paper, and is reponsible for designing, initiating and performing all the experiments presented herein, analyzing their data, and writing the paper itslef. RM assisted performing both the in vivo and ex vivo experiments and helped analysing their data. BL assisted performing the in vivo experiments. PM assisted performing the in vivo experimnts, and analyzing the ex vivo expreiments data. HL assisted performing the in vivo experiments. ER assisted in cells lines preperation and handling, and in analyzing the data. LS assisted performing the in vivo experiments. IR assisted in analyzing the data. ES assisted in performing the in vivo experiments. AB assisted in analyzing the data. SB-E is the principal investigator of the lab and is involved in the design of the experiments, in performing and analyzing their data, and in advising and assisting in writing the paper.
Funding
This work was supported by NIH/NCI Grant # CA125456 and CA172138 (SBE) and by the Israel-USA bi-national Science Foundation # 2005331 (SBE & GGP).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
The Institutional Animal Care and Use Committee of Tel Aviv University approved all studies.
Approval numbers for the experiments
CT26 – P-13-014, MC38 – 10-18-007.
Animal source
BALB/c male and female mice and C57B1/6j male and female mice were purchased from Harlan laboratories (Jerusalem, Israel) at the age of 4 weeks. Animals were housed 3–4 per cage at 22 ± 1 °C, on a 12:12 light/dark cycle and were allowed ad libitum access to food and water. Animals were used at the age of 8–12 weeks. (Animals were age-matched between groups within each experiment.)
Cell line authentication
The CT26 tumor cell line: CT26 tumor cells were kindly provided by Prof. Eliezer Flesher (Department of Human Microbiology, Faculty of Medicine, Tel Aviv University).
The MC38 tumor cell line
The MC38 murine colon adenocarcinoma was derived from tumor that arose in C57B1/6 mice. Tumor cells were kindly provided by Dr. Eran Nizri (Tel Aviv Sourasky Medical center).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J et al (2019) Global cancer observatory: cancer today. 2018 [cited 2019 2/11/2019]; Available from: https://gco.iarc.fr/today
- 2.Morris EJ, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97(7):1110–1118. doi: 10.1002/bjs.7032. [DOI] [PubMed] [Google Scholar]
- 3.Neeman E, Zmora O, Ben-Eliyahu S. A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res. 2012;18(18):4895–4902. doi: 10.1158/1078-0432.CCR-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horowitz M, et al. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12(4):213–226. doi: 10.1038/nrclinonc.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi K, et al. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232(1):58–65. doi: 10.1097/00000658-200007000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Eliyahu S. The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav Immun. 2003;17(Suppl 1):S27–S36. doi: 10.1016/s0889-1591(02)00063-6. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, et al. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res. 1989;49(8):1996–2001. [PubMed] [Google Scholar]
- 8.Shaashua L, et al. Perioperative COX-2 and beta-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res. 2017;23(16):4651–4661. doi: 10.1158/1078-0432.CCR-17-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melamed R, et al. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Wisse E, et al. The pit cell: description of a new type of cell occurring in rat liver sinusoids and peripheral blood. Cell Tissue Res. 1976;173(4):423–435. doi: 10.1007/BF00224305. [DOI] [PubMed] [Google Scholar]
- 11.Kaneda K, Wake K. Distribution and morphological characteristics of the pit cells in the liver of the rat. Cell Tissue Res. 1983;233(3):485–505. doi: 10.1007/BF00212219. [DOI] [PubMed] [Google Scholar]
- 12.Sun H, et al. NK cells in immunotolerant organs. Cell Mol Immunol. 2013;10(3):202–212. doi: 10.1038/cmi.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carson WE, et al. A fatal cytokine-induced systemic inflammatory response reveals a critical role for NK cells. J Immunol. 1999;162(8):4943–4951. [PubMed] [Google Scholar]
- 14.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 15.Sivori S, et al. Comparison of different CpG oligodeoxynucleotide classes for their capability to stimulate human NK cells. Eur J Immunol. 2006;36(4):961–967. doi: 10.1002/eji.200535781. [DOI] [PubMed] [Google Scholar]
- 16.Poeck H, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103(8):3058–3064. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 17.Kwong B, Liu H, Irvine DJ. Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy. Biomaterials. 2011;32(22):5134–5147. doi: 10.1016/j.biomaterials.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guiducci C, et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203(8):1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, et al. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat Immunol. 2019;20(3):265–275. doi: 10.1038/s41590-018-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbett TH, et al. Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35(9):2434–2439. [PubMed] [Google Scholar]
- 21.Sorski L, et al. The impact of surgical extent and sex on the hepatic metastasis of colon cancer. Surg Today. 2014;44(10):1925–1934. doi: 10.1007/s00595-013-0768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorski L et al. (2016) Selective harvesting of marginating-hepatic leukocytes. J Vis Exp, 113 [DOI] [PMC free article] [PubMed]
- 23.Melamed R, et al. The marginating-pulmonary immune compartment in rats: characteristics of continuous inflammation and activated NK cells. J Immunother. 2010;33(1):16–29. doi: 10.1097/CJI.0b013e3181b0b146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89(4):1269–1339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, et al. Isolation of lymphocytes and their innate immune characterizations from liver, intestine, lung and uterus. Cell Mol Immunol. 2005;2(4):271–280. [PubMed] [Google Scholar]
- 26.Agorku DJ, et al. CD49b, CD87, and CD95 are markers for activated cancer-associated fibroblasts whereas CD39 marks quiescent normal fibroblasts in murine tumor models. Front Oncol. 2019;9:716. doi: 10.3389/fonc.2019.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 28.Takeshita F, et al. Cutting edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol. 2001;167(7):3555–3558. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- 29.Ashkar AA, Rosenthal KL. Toll-like receptor 9, CpG DNA and innate immunity. Curr Mol Med. 2002;2(6):545–556. doi: 10.2174/1566524023362159. [DOI] [PubMed] [Google Scholar]
- 30.Krieg AM, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 31.Krug A, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31(7):2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 32.Della Chiesa M, et al. Multidirectional interactions are bridging human NK cells with plasmacytoid and monocyte-derived dendritic cells during innate immune responses. Blood. 2006;108(12):3851–3858. doi: 10.1182/blood-2006-02-004028. [DOI] [PubMed] [Google Scholar]
- 33.Mathan TS, Figdor CG, Buschow SI. Human plasmacytoid dendritic cells: from molecules to intercellular communication network. Front Immunol. 2013;4:372. doi: 10.3389/fimmu.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams DH, Eksteen B, Curbishley SM. Immunology of the gut and liver: a love/hate relationship. Gut. 2008;57(6):838–848. doi: 10.1136/gut.2007.122168. [DOI] [PubMed] [Google Scholar]
- 35.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3(1):51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 36.Lassen MG, et al. Intrahepatic IL-10 maintains NKG2A + Ly49- liver NK cells in a functionally hyporesponsive state. J Immunol. 2010;184(5):2693–2701. doi: 10.4049/jimmunol.0901362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60(6):2109–2117. doi: 10.1002/hep.27254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jinushi M, et al. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology. 2007;120(1):73–82. doi: 10.1111/j.1365-2567.2006.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishikado H, et al. NK cell-depleting anti-asialo GM1 antibody exhibits a lethal off-target effect on basophils in vivo. J Immunol. 2011;186(10):5766–5771. doi: 10.4049/jimmunol.1100370. [DOI] [PubMed] [Google Scholar]
- 40.Victorino F, et al. Tissue-resident NK cells mediate ischemic kidney injury and are not depleted by anti-asialo-GM1 antibody. J Immunol. 2015;195(10):4973–4985. doi: 10.4049/jimmunol.1500651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benish M, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15(7):2042–2052. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glasner A, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184(5):2449–2457. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 43.Clinthorne JF, et al. NK cell maturation and function in C57BL/6 mice are altered by caloric restriction. J Immunol. 2013;190(2):712–722. doi: 10.4049/jimmunol.1201837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldfarb Y, et al. CpG-C oligodeoxynucleotides limit the deleterious effects of beta-adrenoceptor stimulation on NK cytotoxicity and metastatic dissemination. J Immunother. 2009;32(3):280–291. doi: 10.1097/CJI.0b013e31819a2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jahrsdorfer B, Weiner GJ. CpG oligodeoxynucleotides as immunotherapy in cancer. Update Cancer Ther. 2008;3(1):27–32. doi: 10.1016/j.uct.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]