Abstract
Osteonecrosis of the femoral head (ONFH) is a progressive disease that often necessitates hip replacement if hip preservation therapy fails. ONFH places a heavy economic burden and severe psychological pressure on patients. At present, ONFH is treated by either surgical or non‐surgical methods. In clinical practice, stem cells combined with surgery has achieved some positive results, but many problems remain to be resolved. Exosomes are small vesicles of 30–150 nm, which are rich in various nucleic acids, proteins, and small molecules depending on the cells from which they are derived. A growing number of studies have found that exosomes play an important role in tissue damage repair. In comparison with stem cells, exosomes have lower immunogenicity. Also, exosomes can promote cell proliferation and inhibit tumor growth. In addition, exosomes can also be used as natural carriers of drugs. Many studies have shown that exosomes have therapeutic effects in hormone‐induced ONFH. Exosomes have the effect of promoting vascular regeneration and show good application prospects in ONFH. Here, we present a review of studies on the application of exosomes in ONFH to provide a reference for future research.
Keywords: Bone repair mechanism, Exosomes, Mesenchymal stem cells, Osteonecrosis of the femoral head
Exosomes are vesicles secreted by cells into the extracellular space, which are rich in various nucleic acids, proteins, and small molecules depending on the cells from which they are derived. Exosomes have been shown to play roles in many processes, including intercellular signal transduction, tissue damage repair, and inflammatory responses. In comparison with stem cells, exosomes have lower immunogenicity. Exosomes can promote cell proliferation and inhibit tumor growth. In addition, exosomes can also be used as natural carriers of drugs. Many studies have shown that exosomes have therapeutic effects in hormone‐induced ONFH. Exosomes may prevent the progression of ONFH by promoting angiogenesis and osteogenesis.
Introduction
Osteonecrosis of the femoral head (ONFH) is a local abnormal disease of bone metabolism due to abnormal blood supply to the femoral head. The factors that cause the abnormal blood supply to the femoral head can be the direct destruction of mechanics such as the fracture of the femoral neck, or possibly due to the disordered metabolism of substances in the blood circulation. 1 , 2 , 3 This disease usually occurs in young adults, most of whom are within 35–55 years old. 4 Furthermore, this disease is more common in males than in females, and its incidence is increasing. 5 There are more than 30 million patients with ONFH worldwide, with more than eight million patients in China. 6 Therefore, it is the duty and mission of doctors to actively explore the effective treatment means of ONFH.
The pathogenesis of ONFH remains unclear. The most common hypotheses include fat embolism, intraosseous vascular damage, and intraosseous hypertension. 7 , 8 , 9 Although the etiology and pathogenesis of ONFH remain unclear, the pathological changes are consistent. When osteocytes and stromal cells of the femoral head die, the bone repair mechanism is activated. The process of ONFH repair can be divided into limited repair, destructive repair, and reconstructive repair. 10 Many treatments are available for ONFH, but there is no standard therapy. ONFH seriously affects a patient's ability to work and their quality of life and is accompanied by a heavy psychological burden.
At present, the most commonly used staging system for ONFH is the Association Research Circulation Osseous (ARCO) staging method, which is divided into four stages. 11 Surgical and non‐surgical treatment options are available for early ONFH (ARCO stage I and II). 3 , 12 During these stages, the goal is to preserve femoral head function to the maximum extent, prevent further progression of the disease, and delay or prevent joint replacement. Advanced ONFH (ARCO stage III and IV) generally requires joint replacement. 3 , 13 It is of great clinical significance to explore active and effective means of hip preservation and delay or even reverse the disease process according to the early stage of ONFH. With the development of medical technologies and improved understanding of ONFH, many new methods and techniques have been applied for the treatment of this disease.
During the normal repair of bone, there is a balance between osteoblasts and osteoclasts. When the cell function is abnormal, this will cause the balance of bone resorption and bone repair to be broken, so that the femoral head structure is abnormal. Karasuyama et al. found that the number of osteoclasts at the junction of necrotic area and normal area after femoral head collapse was significantly higher than that without collapse. 14 Li et al. found that in the middle and late stage of femoral head necrosis, osteoclasts gathered in the repair reaction area between the necrotic area and the normal area, which is the common site of fracture line. 15 Yu et al. found that in patients with femoral head necrosis, the angiogenesis activity and apoptosis activity of bone microvascular endothelial cells were changed, which showed that the angiogenesis activity decreased and the apoptosis increased. 16 Therefore, promoting vascular regeneration, increasing osteogenesis, and inhibiting bone resorption is an effective strategy for treating ONFH. Many studies have confirmed that the apoptosis and abnormal differentiation of bone cells and bone marrow stem cells are also one of the important reasons for the progression of femoral head necrosis. 17 After the bone necrosis of the femoral head occurs, the number of internal mesenchymal stem cells decreases and the osteogenic differentiation function weakens. Therefore, mesenchymal stem cell transplantation becomes a promising option for the treatment of this disease. The most common sources of stem cells for ONFH are bone marrow mesenchymal stem cells (BMSCs), fat tissue‐derived mesenchymal stem cells, embryonic‐derived mesenchymal stem cells, peripheral blood hematopoietic stem cells. 18 , 19 , 20 , 21 However, it was found that bone marrow mesenchymal stem cells from patients with femoral head necrosis showed decreased proliferation and increased apoptosis in vitro. 22 Despite the many achievements of stem cell therapy, a number of problems remain to be resolved in terms of the underlying molecular mechanism of stem cell differentiation, the survival rate after stem cell implantation, patient inclusion and exclusion criteria, and optimal transplantation methods.
Studies have shown that mesenchymal stem cells play a role through their paracrine function. Exosomes are cell‐secreted vesicles 30–150 nm in diameter that contain complex mixtures of nucleic acids, proteins, and small molecules. 23 Exosomes were initially considered to be a means for cells to dispose of waste. However, subsequent studies showed that exosomes have an important effect on intercellular signal transduction, tissue angiogenesis, and inflammatory responses. The proteins in exosomes may mediate their specific binding to target cells and may be related to cell signal transduction, playing various roles in physiological and pathological processes, such as immunization, tumors and so on. Exosomes contain a variety of substances derived from cells, which can participate in the information exchange to recipient cells and regulate their functions. It was found that the mechanism by which mesenchymal stem cells exert their effects is through their paracrine effects. The MSC‐derived exosomes play a similar role. At present, the application of exosomes in the treatment of ONFH is mostly at the stage of basic research. Here, we searched the PUBMED and MEDLINE databases using the keywords “exosomes” or “osteonecrosis” or “ONFH” to review studies on the role of exosomes in ONFH and thus provide a reference for further studies.
Characterization, Extraction, and Identification of Exosomes
Characterization of Exosomes
Exosomes are small vesicles, which vary in morphology and usually appear as hemispherical structures with lipid bilayers on electron microscopy. 24 , 25 , 26 , 27 Exosomes contain various small molecules, nucleic acids, and proteins and have different receptors on their surfaces depending on their origin. Many different proteins are present in exosomes, including annexins associated with intercellular membrane fusion and RAS‐related GTP‐binding proteins (RAB proteins), protein kinases and heterotrimer G proteins associated with signal transduction, and metabolism‐related enzymes. 28 In addition, members of the tetraspanin superfamily are abundant in exosomes and can interact with major histocompatibility complex molecules and integrins. 29 Furthermore, exosomes are rich in cholesterol and sphingomyelin. Due to their special mode of formation, exosomes can highly express cellular endogenous proteins. The compositions of exosomes differ according to the cells from which they are derived.
Extraction of Exosomes
High purity exosome enrichment is a key step in basic exosome research and for further clinical applications. At present, there are many techniques for the separation and purification of exosomes, and they are constantly improving. Common methods include ultra‐centrifugation, sucrose gradient separation, precipitation, the size exclusion method (SEC), ultrafiltration, the microfluidic chip method, and the surface composition affinity separation method. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 However, most methods have the disadvantages of low extraction efficiency and difficult to popularize.
Identification of Exosomes
Cells can secrete a wide variety of components into the external space. Extracellular vesicles can be divided into three types according to the mechanism and size of release: exosomes, micro‐vesicles, and apoptotic bodies, while exosomes contain the characteristic surface protein from endosomal pathway origin. 38 Using these surface proteins can identify and isolate exosomes from other micro‐vesicles, apoptotic bodies. Each exosome has a unique lipid composition compared to other vesicle types, as they are rich in cholesterol and diacylglycerol. Currently, the identification method used for external urology mainly conducts from three aspects: size, morphology and surface marker. 39 Exosome size is determined by dynamic light scattering analysis and nanoparticle tracking analysis. 40 , 41 Morphological analysis is performed mainly by transmission electron microscopy.26 Western blotting is the main method used for surface marker identification, which has demonstrated the presence of CD9, CD63, CD81on the exosome surface. Gene chip and high‐throughput sequencing technologies can be used to detect nucleic acids for subsequent analysis, while protein components are analyzed by flow cytometry and protein chip methods. Cell co‐culture systems were constructed to examine the effects of exosomes on target cell function and related signaling pathways. 42 , 43 , 44
Roles of Exosomes in ONFH
Therapeutic Mechanism of Action of Exosomes
Exosomes Can Enhance the Proliferation and Antiapoptotic Activity of BMSCs
BMSCs are derived from the mesoderm and ectoderm at the early stage of development and have multidirectional differentiation potential. Giuliana et al. injected BMSCs into animals with unilateral gastrocnemius muscle injury through venous system and found that the transplanted BMSCs can migrate to the damaged gastrocnemius muscle tissue and repair the damaged muscle. 45 Yavuzer reported that transplantation of autogenous BMSCs combined with fibrin glue into the area of tendon injury in a rabbit model immediately after the injury promoted the early histomorphological and biomechanical repair of the tendon. 46 BMSCs are excellent seed cells and have been widely used in bone tissue engineering due to their multidirectional differentiation potential and the ease of their extraction and acquisition of immunogenicity. However, the proliferation and osteogenic potential of BMSCs decrease with age. And the viability of mesenchymal stem cells decreased after implantation. Exosomes have also been used as drug carriers. 47 Guo et al. reported that BMSCs integrated with exosomes derived from human synovium‐derived mesenchymal stem cells (MSCs) showed enhanced proliferation and antiapoptotic effects. Although further studies are required to determine the precise mechanism of these effects, the mechanism may involve promotion of β‐catenin nuclear translocation. 48
Ability of Exosomes to Promote Bone Formation and Blood Vessel Formation
It was found that the mechanism by which mesenchymal stem cells exert their effects is through their paracrine effects. The MSC‐derived exosomes play a similar role. Researcher found that exosomes derived from BMSCs have potential roles in the treatment of a variety of diseases. Li et al. reported that exosomes from both normal BMSCs and those from patients with ONFH can promote osteogenesis and angiogenesis, although those from ONFH patients showed reduced osteogenic and angiogenic capabilities. 49 Liu et al. reported that exosomes derived from human‐induced pluripotent stem cell‐derived MSCs may promote angiogenesis by activating the PI3K/Akt signaling pathway. 50 When phosphorylated, Erk and Akt activate the angiogenic signaling pathway. Akt can induce antiapoptotic signaling, 51 and therefore the Akt pathway is considered to be the main survival pathway in endothelial cells. Platelet‐rich plasma‐derived exosomes promote angiogenesis by activating Akt and Erk signaling pathways. 52 Yeh et al. confirmed that both hormones and ethanol reduce the expression of osteogenic genes and proteins via Wnt signaling‐related genes, while increasing the expression of fat formation‐related genes and proteins. Platelet‐rich plasma‐derived exosomes were shown to enhance osteogenic protein expression via the Wnt/catenin signaling pathway. In endoplasmic reticulum stress, platelet‐rich plasma derived exosomes block CHOP mediated inhibition of Bcl‐2 protein expression through Akt/bad/Bcl‐2/caspase‐3 signaling pathway. 52 Xu et al. reported that miR‐224‐3p was downregulated, while focal adhesion kinase family interacting protein of 200 kDa (FIP200) was upregulated in ONFH. Lower miR‐224‐3p levels in exosomes can promote angiogenesis by promoting the proliferation, migration, and invasion of vascular endothelial cells, thereby preventing ONFH. 53 The CD41/integrin 3‐fak–PI3K/Akt–Runx2 pathway is an important pathway of osteogenic differentiation of MSCs. CD41‐affluent normal exosomes can promote the osteogenic differentiation of MSCs. 42 Exosomes derived from human CD34+ stem cells transfected with miR‐26a can reverse the pathogenic process of hormone‐induced ONFH by increasing vascular density and trabecular integrity. 54 In addition, Li et al. reported that plasminogen activator inhibitor 1 (PAI‐1), which is obviously malformed in the femoral head area, may be the cause of idiopathic ONFH susceptibility. 55 BMSC‐derived exosomes promote the expression of vascular cell PAI‐1 in the local microenvironment of femoral head necrosis in rabbits, thus providing a new perspective on the etiology of femoral head necrosis. 43 (Table 1) (Fig. 1).
TABLE 1.
Roles of exosomes derived from different MSCs in ONFH
| Author | Cell type | Animal | Type of ONFH | Separation method | Exosome diameter | Biological effect | Mechanism of action |
|---|---|---|---|---|---|---|---|
| Li et al. 49 |
Normal femoral derived BMSC Necrotic femoral head derived BMSC |
SD rats | Steroid‐induced ONFH | Ultrafiltration | 40–150 nm | Attenuated pro‐osteogenic and pro‐angiogenic effects of sEVs derived from BMSCs | Activation of Wnt/β‐catenin pathway promotes osteogenic differentiation |
| Liu et al. 50 | Human‐induced pluripotent stem cell‐derived MSCs | SD rats | Steroid‐induced ONFH |
Ultrafiltration |
30–100 nm | Promotion of local angiogenesis and prevention of bone loss | Activation of the PI3K/Akt signaling pathway in endothelial cells |
| Tao et al. 52 | Human platelet‐rich plasma | SD rats | Steroid‐induced ONFH |
Ultrafiltration |
30–100 nm | Prevention of GC‐induced apoptosis | Promotion of Bcl‐2 expression via the Akt/Bad/Bcl‐2 signaling pathway in ER stress |
| Xu et al. 53 | BMSCs | SD rats | Traumatic ONFH |
Ultrafiltration Extraction kits |
30–100 nm | Promotion of angiogenesis | Lower miR‐224‐3p levels in exosomes derived from BMSCs promote angiogenesis of traumatic ONFH by upregulating FIP200 |
| Zhu et al. 42 | ONFH bone tissues | rats | Steroid‐induced ONFH | ‐ | 117.3 ± 41.9 nm in NOR‐exos and 131.9 ± 46.6 nm in ONFH‐exos | Downregulation of CD41 could impair osteogenic differentiation and migration of MSCs | CD41/integrin β3‐FAK‐Akt‐Runx2 pathway |
| Zuo et al. 54 | Human CD34+ stem cells transfected with miR‐26a | SD rats | Steroid‐induced ONFH | Ultracentrifugation | 40–150 nm | Promotion of angiogenesis and osteogenesis | Pro‐angiogenic effect of CD34+‐exos and the pro‐osteogenic effect of miR‐26a were combined in miR‐26a‐CD34+‐exos and functioned cooperatively in reversing the pathogenic process of GC‐induced ONFH |
| Li et al. 55 | BMSCs | Rabbits | Steroid‐induced ONFH | Exosome concentration kit | 30–150 nm | Promotion of angiogenesis | BMSC exosomal miR‐mediated upregulation of the fibrinolytic regulator, PAI‐1, in vascular cells |
Abbreviations: BMSC, bone marrow mesenchymal stem cell; exos, exosomes; miR, microRNA; MSC, mesenchymal stem cell; ONFH, osteonecrosis of the femoral head; sEVs, small extracellular vesicles.
Fig. 1.
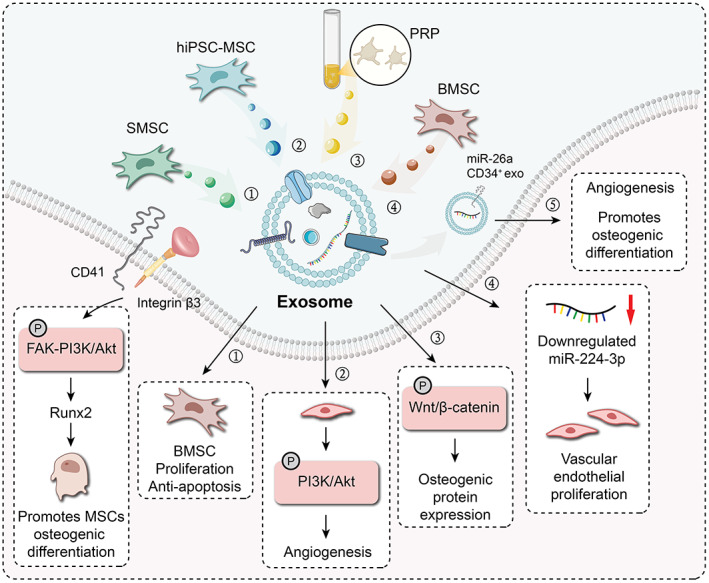
The exosomes from different tissues and their mechanisms that promote osteogenesis and promote angiogenesis ① SMSC‐derived exosomes can enhance the proliferation and anti‐apoptotic capacity of BMSC; ② hiPSC‐MSC‐derived exosomes promote angiogenesis by activating the PI3K/Akt signaling pathway in vascular endothelial cells; ③ Platelet‐rich plasma‐derived exosomes enhance osteobinin expression levels through the Wnt/‐catenin signaling pathway; ④ BMSC‐derived exosomes containing downregulated levels of miR‐224‐3p can promote the proliferation of vascular endothelial cells; ⑤ miR‐26a‐CD34+ exosomes can promote angiogenesis and promote osteogenesis; Normal conditional exosomes (NOR‐exos) promote the osteogenic differentiation of the MSC through activating the CD41/integrin β3‐FAK‐PI3K/Akt‐Runx2 pathway
Diagnostic Value of Exosomes
Early detection, early diagnosis, and early treatment are important principles in disease management. In clinical practice, patients with ONFH often seek medical treatment due to hip discomfort, and the diagnosis is usually ARCO stage II or above. Furthermore, the diagnosis relies mainly on MRI and CT. Early ONFH is often asymptomatic, and there are no sensitive or specific hematological indicators. Therefore, it is necessary to find a convenient laboratory test for early prediction of the occurrence and development of ONFH. Exosomes are widely present in the pleuroperitoneal and pleural effusions of blood and urine, with high levels in body fluids, and the number of exosomes in each microliter of serum can reach up to 3 × 106. 56 Compared with traditional circulatory markers, exosomes are stable and have a longer half‐life in body fluids. Compared with samples required for histopathological examination, body fluid specimens can be obtained easily, less invasively, and repeatedly. Exosomes have been increasingly used in disease diagnosis, with most efforts focusing on tumor‐related diseases. Due to the characteristics outlined above, exosomes are expected to be useful as new circulating biomarkers with broad applicability to assist in the diagnosis of clinical diseases.
During the early stages of ONFH, changes in the local microenvironment and cell function may be found in blood. Zhu et al. demonstrated that serum exosome levels were significantly lower in patients with steroid‐induced ONFH and hormone‐induced ONFH compared with healthy controls. 57 Receiver operating characteristic curve analysis showed that serum exosomes have moderate diagnostic accuracy for hormonal ONFH. Furthermore, through the early screening of the serum exosome level of patients with suspected steroid induced‐femoral head necrosis, this allowed identification of patients that may have femoral head necrosis. This was confirmed by definite diagnosis using CT and MRI in patients with a history of hormone treatment. These observations demonstrated the importance of the early detection of lesions in the femoral head.
Conclusion
In the past decade, exosomes, as a cell‐free therapy, have achieved positive results in the research of many diseases. This article summarizes the therapeutic effects of exosomes from different tissue and cell sources in osteonecrosis of the femoral head.
The main strategy of repair and regeneration of osteonecrosis of the femoral head is to promote vascular regeneration and bone regeneration. At present, the research on exosomes mainly focuses on basic research. It has been found that exosomes derived different mesenchymal stem cells can promote angiogenesis and bone repair through a variety of signal pathways. Osteonecrosis of the femoral head is mainly caused by trauma, hormones and alcohol. At present, most animal models are hormone induced necrosis models, and exosomes are mainly applied by caudal vein injection. However, whether this application can make the exosomes reach the necrotic part and play a role needs further examination. At the same time, the interaction between exosomes and mesenchymal stem cells, osteoblasts and osteoclasts in necrotic areas needs to be further explored. We believe that with the in‐depth study, it will further reveal the mechanism of osteonecrosis of the femoral head, so as to provide more references for the treatment of the disease.
Funding
This study was funded by the National Natural Science Foundation of China (81572148, 82001481), Bethune Charitable Foundation (G‐X‐2019‐1107‐8). Key Clinical Projects of Peking University Third Hospital (No.BYSYZD2019037).
Authors' Contributions
Jiang Peng and Hua Tian conceived the project. Haoye Meng, Sida Liao, Jian Zhang, and Yanjun Guan conducted the article search and acquisition. Xiuzhi Liu and Cheng Wang wrote the manuscript. All authors approved the final manuscript.
Contributor Information
Hua Tian, Email: tianhua@bjmu.edu.cn.
Jiang Peng, Email: pengjiang301@126.com.
References
- 1. Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rayadhyaksha A, et al. Osteonecrosis of the hip: management in the twenty‐first century. J Bone Jt Surg. 2002;84(5):834–53. [Google Scholar]
- 2. Mont MA, Ragland PS, Etienne G. Core decompression of the femoral head for osteonecrosis using percutaneous multiple small‐diameter drilling. Clin Orthop Relat Res. 2004;429:131–8. [DOI] [PubMed] [Google Scholar]
- 3. Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Jt Surg Am. 2006;88(5):1117–32. [DOI] [PubMed] [Google Scholar]
- 4. Kuang MJ, Huang Y, Zhao XG, Zhang R, Ma JX, Wang DC, et al. Exosomes derived from Wharton's jelly of human umbilical cord mesenchymal stem cells reduce osteocyte apoptosis in glucocorticoid‐induced osteonecrosis of the femoral head in rats via the miR‐21‐PTEN‐AKT signalling pathway. Int J Biol Sci. 2019;15(9):1861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chinese guideline for the diagnosis and treatment of osteonecrosis of the femoral head in adults. Orthop Surg. 2017;9(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao DW, Yu M, Hu K, Wang W, Yang L, Wang BJ, et al. Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: results from a nationally representative survey. Chin Med J. 2015;128(21):2843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hungerford DS. Pathogenesis of ischemic necrosis of the femoral head. Instr Course Lect. 1983;32:252–60. [PubMed] [Google Scholar]
- 8. Jones JP Jr, Sakovich L. Fat embolism of bone. A roentgenographic and histological investigation, with use of intra‐arterial lipiodol, in rabbits. J Bone Jt Surg Am. 1966;48(1):149–64. [PubMed] [Google Scholar]
- 9. Kawai K, Tamaki A, Hirohata K. Steroid‐induced accumulation of lipid in the osteocytes of the rabbit femoral head. A histochemical and electron microscopic study. J Bone Jt Surg Am. 1985;67(5):755–63. [PubMed] [Google Scholar]
- 10. Plenk H Jr, Gstettner M, Grossschmidt K, Breitenseher M, Urban M, Hofmann S. Magnetic resonance imaging and histology of repair in femoral head osteonecrosis. Clin Orthop Relat Res. 2001;386:42–53. [DOI] [PubMed] [Google Scholar]
- 11. Yoon BH, Mont MA, Koo KH, Chen CH, Cheng EY, Cui Q, et al. The 2019 revised version of association research circulation osseous staging system of osteonecrosis of the femoral head. J Arthroplasty. 2020;35(4):933–40. [DOI] [PubMed] [Google Scholar]
- 12. Petrigliano FA, Lieberman JR. Osteonecrosis of the hip: novel approaches to evaluation and treatment. Clin Orthop Relat Res. 2007;465:53–62. [DOI] [PubMed] [Google Scholar]
- 13. Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, et al. Osteonecrosis of the hip: management in the 21st century. Instr Course Lect. 2003;52:337–55. [PubMed] [Google Scholar]
- 14. Karasuyama K, Yamamoto T, Motomura G, Sonoda K, Kubo Y, Iwamoto Y. The role of sclerotic changes in the starting mechanisms of collapse: a histomorphometric and FEM study on the femoral head of osteonecrosis. Bone. 2015;81:644–8. [DOI] [PubMed] [Google Scholar]
- 15. Li W, Sakai T, Nishii T, Nakamura N, Takao M, Yoshikawa H, et al. Distribution of TRAP‐positive cells and expression of HIF‐1alpha, VEGF, and FGF‐2 in the reparative reaction in patients with osteonecrosis of the femoral head. J Orthop Res. 2009;27(5):694–700. [DOI] [PubMed] [Google Scholar]
- 16. Yu H, Liu P, Zuo W, Sun X, Liu H, Lu F, et al. Decreased angiogenic and increased apoptotic activities of bone microvascular endothelial cells in patients with glucocorticoid‐induced osteonecrosis of the femoral head. BMC Musculoskelet Disord. 2020;21(1):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng H, Yang E, Peng H, Li J, Chen S, Zhou J, et al. Gastrodin prevents steroid‐induced osteonecrosis of the femoral head in rats by anti‐apoptosis. Chin Med J. 2014;127(22):3926–31. [PubMed] [Google Scholar]
- 18. Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. [DOI] [PubMed] [Google Scholar]
- 19. Pak J, Lee JH, Jeon JH, Lee SH. Complete resolution of avascular necrosis of the human femoral head treated with adipose tissue‐derived stem cells and platelet‐rich plasma. J Int Med Res. 2014;42(6):1353–62. [DOI] [PubMed] [Google Scholar]
- 20. Hwang YS, Polak JM, Mantalaris A. In vitro direct osteogenesis of murine embryonic stem cells without embryoid body formation. Stem Cells Dev. 2008;17(5):963–70. [DOI] [PubMed] [Google Scholar]
- 21. Aarvold A, Smith JO, Tayton ER, Jones AM, Dawson JI, Lanham S, et al. From bench to clinic and back: skeletal stem cells and impaction bone grafting for regeneration of bone defects. J Tissue Eng Regener Med. 2014;8(10):779–86. [DOI] [PubMed] [Google Scholar]
- 22. Houdek MT, Wyles CC, Packard BD, Terzic A, Behfar A, Sierra RJ. Decreased osteogenic activity of mesenchymal stem cells in patients with corticosteroid‐induced osteonecrosis of the femoral head. J Arthroplasty. 2016;31(4):893–8. [DOI] [PubMed] [Google Scholar]
- 23. Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30:3–22. [DOI] [PubMed] [Google Scholar]
- 24. Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166(1):189–97. [PubMed] [Google Scholar]
- 25. De Broe ME, Wieme RJ, Logghe GN, Roels F. Spontaneous shedding of plasma membrane fragments by human cells in vivo and in vitro. Clin Chim Acta. 1977;81(3):237–45. [DOI] [PubMed] [Google Scholar]
- 26. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. [DOI] [PubMed] [Google Scholar]
- 27. Weidle UH, Birzele F, Kollmorgen G, Rüger R. The multiple roles of exosomes in metastasis. Cancer Genomics Proteomics. 2017;14(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yunusova NV, Tugutova EA, Tamkovich SN, Kondakova IV. The role of exosomal tetraspanins and proteases in tumor progression. Biomed Khim. 2018;64(2):123–33. [DOI] [PubMed] [Google Scholar]
- 29. Faught E, Henrickson L, Vijayan MM. Plasma exosomes are enriched in Hsp70 and modulated by stress and cortisol in rainbow trout. J Endocrinol. 2017;232(2):237–46. [DOI] [PubMed] [Google Scholar]
- 30. Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, et al. New mechanism for notch signaling to endothelium at a distance by delta‐like 4 incorporation into exosomes. Blood. 2010;116(13):2385–94. [DOI] [PubMed] [Google Scholar]
- 31. Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles. 2014;18(3):v24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015(4):319–23. [DOI] [PubMed] [Google Scholar]
- 34. Momen‐Heravi F, Balaj L, Alian S, Trachtenberg AJ, Hochberg FH, Skog J, et al. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol. 2012;3:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sabapatha A, Gercel‐Taylor C, Taylor DD. Specific isolation of placenta‐derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56(5–6):345–55. [DOI] [PubMed] [Google Scholar]
- 36. Wang Z, Wu HJ, Fine D, Schmulen J, Hu Y, Godin B, et al. Ciliated micropillars for the microfluidic‐based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13(15):2879–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic device (ExoChip) for on‐chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14(11):1891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lässer C, Eldh M, Lötvall J. Isolation and characterization of RNA‐containing exosomes. J Visualized Exp. 2012;59:e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Szatanek R, Baj‐Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. 2017;18(6):1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine. 2011;7(6):780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu W, Guo M, Yang W, Tang M, Chen T, Gan D, et al. CD41‐deficient exosomes from non‐traumatic femoral head necrosis tissues impair osteogenic differentiation and migration of mesenchymal stem cells. Cell Death Dis. 2020;11(4):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liao W, Ning Y, Xu HJ, Zou WZ, Hu J, Liu XZ, et al. BMSC‐derived exosomes carrying microRNA‐122‐5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin Sci. 2019;133(18):1955–75. [DOI] [PubMed] [Google Scholar]
- 44. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cell. 2019;8(4):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ferrari G, Cusella‐De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow‐derived myogenic progenitors. Science. 1998;279(5356):1528–30. [DOI] [PubMed] [Google Scholar]
- 46. Yavuzer R, Tuncer S, Başterzi Y, Işik I, Sari A, Latifoğlu O. Reconstruction of orbital floor fracture using solvent‐preserved bone graft. Plast Reconstr Surg. 2004;113(1):34–44. [DOI] [PubMed] [Google Scholar]
- 47. Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31(5):543–51. [DOI] [PubMed] [Google Scholar]
- 48. Guo SC, Tao SC, Yin WJ, Qi X, Sheng JG, Zhang CQ. Exosomes from human synovial‐derived mesenchymal stem cells prevent glucocorticoid‐induced osteonecrosis of the femoral head in the rat. Int J Biol Sci. 2016;12(10):1262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li J, Ge Z, Ji W, Yuan N, Wang K. The proosteogenic and proangiogenic effects of small extracellular vesicles derived from bone marrow mesenchymal stem cells are attenuated in steroid‐induced osteonecrosis of the femoral head. Biomed Res Int. 2020;2020:4176926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu X, Li Q, Niu X, Hu B, Chen S, Song W, et al. Exosomes secreted from human‐induced pluripotent stem cell‐derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int J Biol Sci. 2017;13(2):232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ho L, Tan SY, Wee S, Wu Y, Tan SJ, Ramakrishna NB, et al. ELABELA is an endogenous growth factor that sustains hESC self‐renewal via the PI3K/AKT pathway. Cell Stem Cell. 2015;17(4):435–47. [DOI] [PubMed] [Google Scholar]
- 52. Tao SC, Yuan T, Rui BY, Zhu ZZ, Guo SC, Zhang CQ. Exosomes derived from human platelet‐rich plasma prevent apoptosis induced by glucocorticoid‐associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl‐2 signal pathway. Theranostics. 2017;7(3):733–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu HJ, Liao W, Liu XZ, Hu J, Zou WZ, Ning Y, et al. Down‐regulation of exosomal microRNA‐224‐3p derived from bone marrow‐derived mesenchymal stem cells potentiates angiogenesis in traumatic osteonecrosis of the femoral head. FASEB J. 2019;33(7):8055–68. [DOI] [PubMed] [Google Scholar]
- 54. Zuo R, Kong L, Wang M, Wang W, Xu J, Chai Y, et al. Exosomes derived from human CD34(+) stem cells transfected with miR‐26a prevent glucocorticoid‐induced osteonecrosis of the femoral head by promoting angiogenesis and osteogenesis. Stem Cell Res Ther. 2019;10(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li L, Wang Y, Yu X, Bao Y, An L, Wei X, et al. Bone marrow mesenchymal stem cell‐derived exosomes promote plasminogen activator inhibitor 1 expression in vascular cells in the local microenvironment during rabbit osteonecrosis of the femoral head. Stem Cell Res Ther. 2020;11(1):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–8. [DOI] [PubMed] [Google Scholar]
- 57. Zhu HY, Gao YC, Wang Y, Zhang CQ. Circulating exosome levels in the diagnosis of steroid‐induced osteonecrosis of the femoral head. Bone Jt Res. 2016;5(6):276–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


