Figure 6.
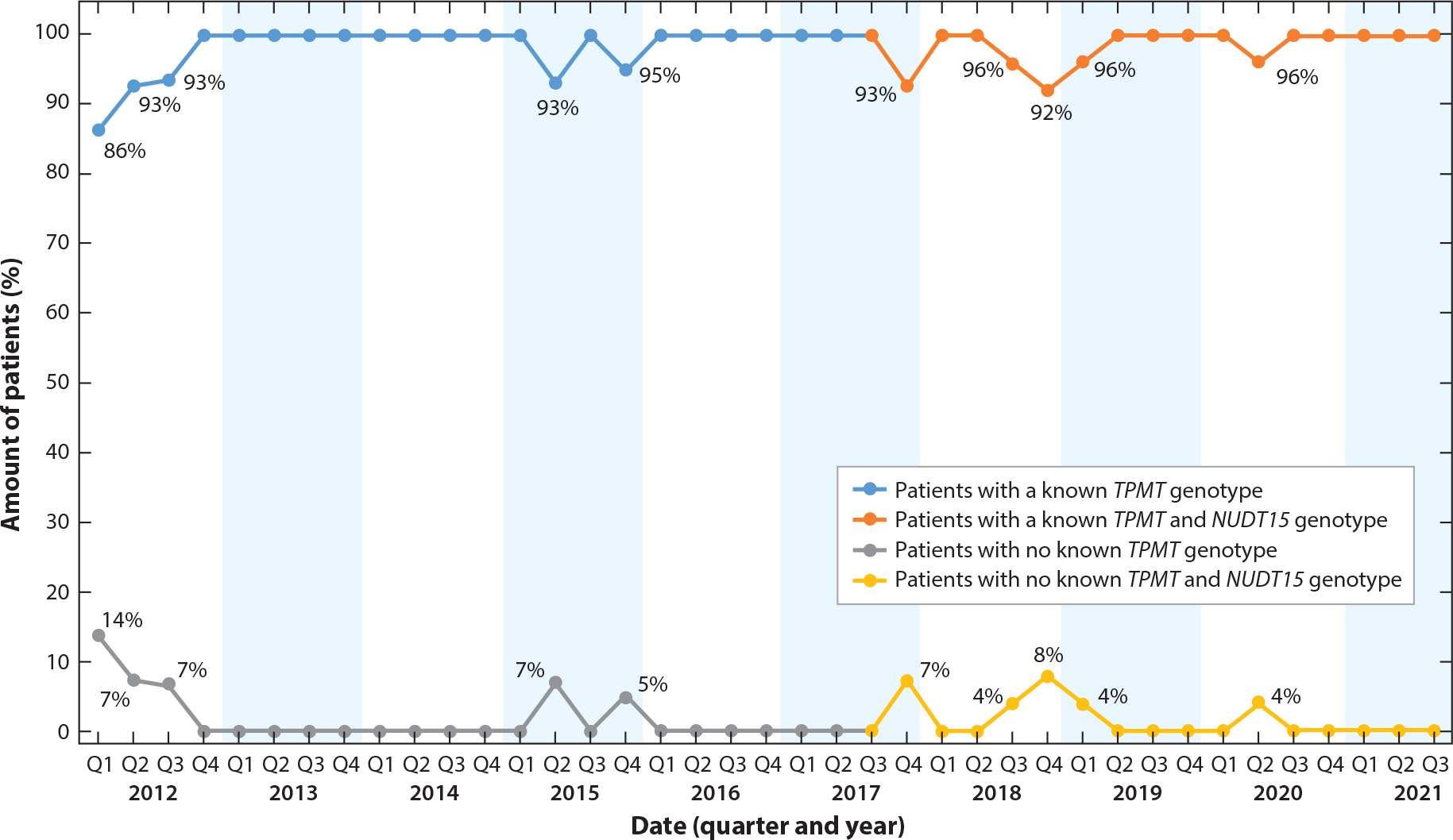
Percentage of thiopurine-naive patients diagnosed with acute lymphocytic leukemia who had a known TPMT and NUDT15 genotype prior to initiating thiopurine therapy at St. Jude Children’s Research Hospital, by quarter. TPMT genotyping was implemented prior to 2012; NUDT15 genotyping was implemented clinically in 2017. The goal quality metric is 100% for the top curve and 0% for the bottom curve. Approximately 22 patients per quarter were included in this metric.
