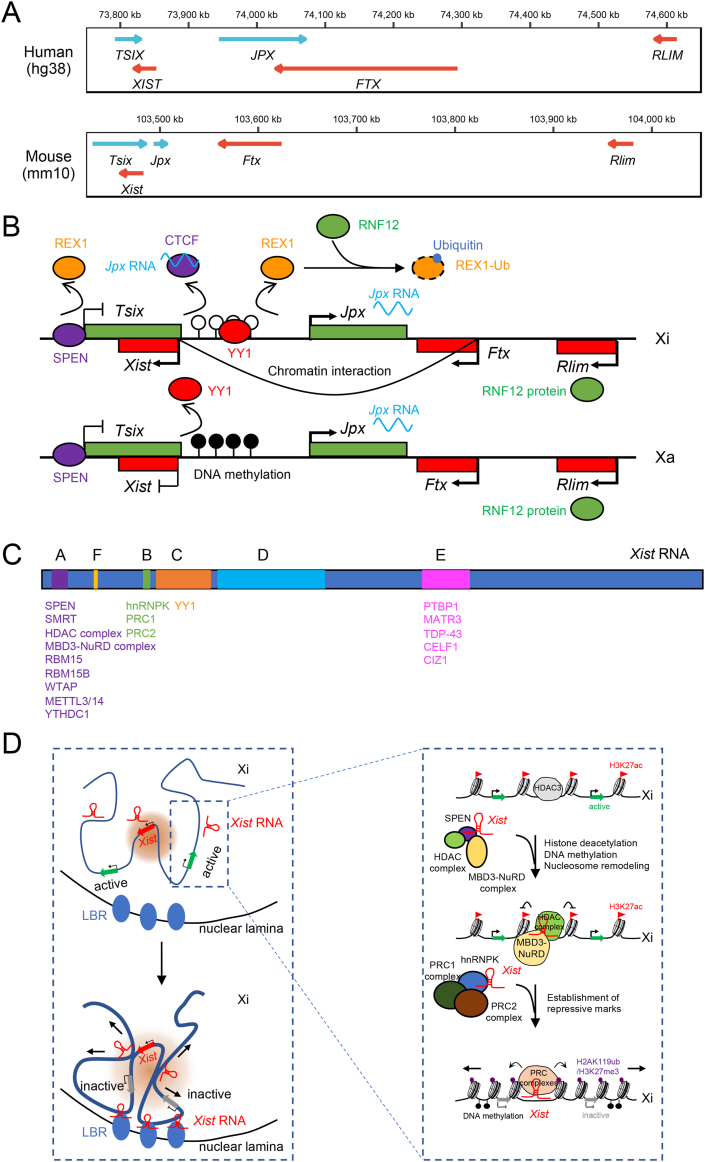
Figure 1.
The long noncoding Xist RNA and its roles in X chromosome inactivation. (A) Genomic arrangement of XIST/Xist and its regulators in X inactivation center (XIC) in human and mouse. (B) Factors involved in the transcriptional activation of mouse Xist. Jpx RNA transcribed from both X chromosomes interacts with CTCF insulator to release it from the Xist regulatory region. YY1 competes with REX1 repressor to bind the Xist regulatory region on Xi lacking DNA methylation, while the methylated copy is not bound, achieving selective activation of Xist. The repressor REX1 is further recognized by the E3 ubiquitin ligase RNF12, encoded by Rlim in XIC, leading to the ubiquitination and degradation of REX1. Ftx promotes Xist transcription through nuclear proximity of Xist and Ftx loci, independently of Ftx transcripts. However, active Ftx transcription is required for Xist accumulation. SPEN remodels the chromatin to silence Tsix and activate Xist. (C) Overview of repeat motifs in mouse Xist RNA. The proteins or complexes interacting with these repeats are indicated below. (D) The roles of Xist in the establishment of random XCI. As shown in the left panel, spatially, Xist RNA binds some locally confined loci on the Xi and nucleates local protein gradients to form SMACs (shown as light red circles). Xist RNA is tethered to the inactive X nucleation center by YY1. The Xi is recruited to the nuclear lamina through the Xist-LBR interaction, and the SMACs gradually expand to silence the whole X chromosome. Arrows indicate the expansion of the complex and the spreading of gene silencing on Xi. In the right panel, enlarged view of Xist-mediated chromatin dynamics near gene loci on Xi during XCI is shown. Xist RNA interacts with SPEN and further activates HDAC and MBD3-NuRD complexes, enabling removal of active histone marks, remodeling of nucleosomes, and DNA methylation. Xist also recruits PRC1 and PRC2 through hnRNPK to establish repressive histone marks, such as H2AK119ub and H3K27me3.