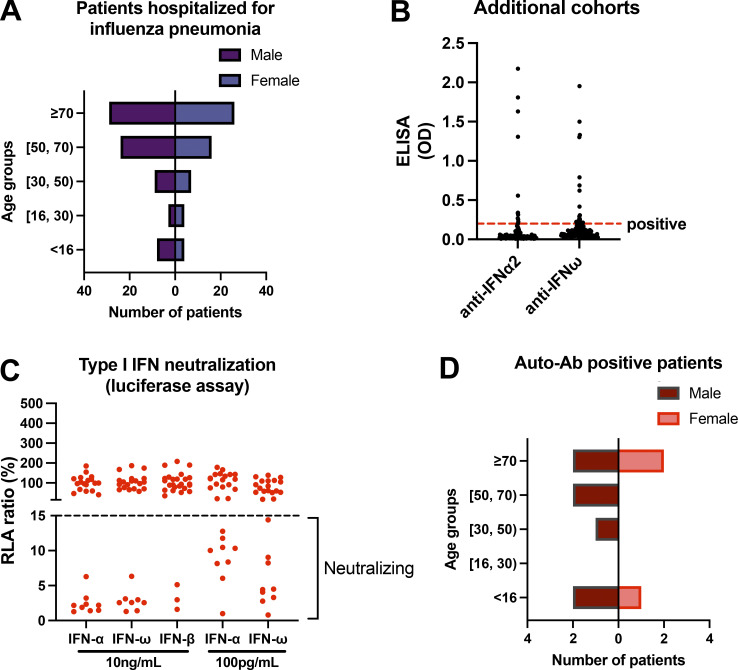
Figure 6.
Auto-Abs neutralizing IFN-α2 and/or IFN-ω in ELISA-positive patients hospitalized with influenza pneumonia in additional cohorts. (A) Age and sex distribution of patients from Chile, Spain, France, Belgium, and Taiwan hospitalized for influenza pneumonia (n = 130). (B) Patient plasma samples were tested by ELISA for auto-Abs against IFN-α2 and -ω. Patient plasma samples were diluted 1:50 before being added to plates coated with 2 μg/ml rhIFN-α or rhIFN-ω. HRP-conjugated goat antiserum against human IgG or IgA was added to final concentration of 2 μg/ml. OD was measured. Each plasma sample was tested once. (C) Luciferase-based neutralization assay to detect auto-Abs neutralizing 10 ng/ml or 100 pg/ml IFN-α2, IFN-ω, or IFN-β. Plasma samples from ELISA-positive patients were diluted 1:10 in all tests. HEK293T cells were transfected with the dual luciferase system with IFN-sensitive response elements (ISRE) before treatment with type I IFNs with or without plasma from patients, and relative luciferase activity (RLA) was calculated by normalizing firefly luciferase activity against Renilla luciferase activity. An RLA <15% the value for the mock treatment was considered to indicate that the antibodies were neutralizing (dashed line). (D) Age and sex distribution of patients with auto-Ab neutralizing IFN-α2 and/or IFN-ω (n = 10).