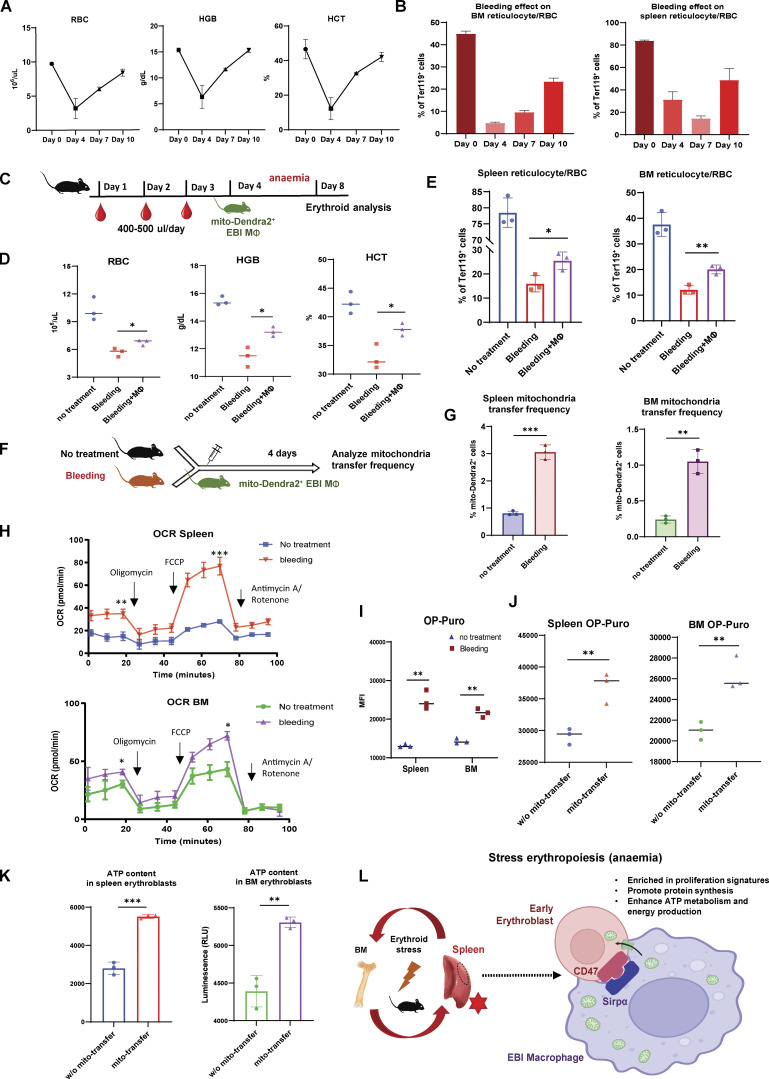
Figure 7.
Mitochondria transfer from EBI macrophages ameliorates erythroid stress from serial blood withdrawal. (A) Peripheral blood analysis of RBC, HGB, and HCT levels of mice after serial bleeding. (B) FACS analysis of erythroid fraction in spleen and BM of mice after serial bleeding. (C) Schematic illustration of the experiment to analyze the effect of EBI macrophage infusion on erythroid recovery following serial bleeding. (D and E) Peripheral blood analysis (D) and FACS analysis (E) of erythroid fraction in spleen and BM of mice treated with the indicated conditions. (F) Schematic illustration of the experiment to assess mitochondria transfer frequency in response to serial bleeding. (G) Frequency of mitochondria transfer, as indicated by the percentage of mito-Dendra2+ events among early erythroblasts, in the spleens and BM of untreated or serially bled mice infused with EBI macrophages from mito-Dendra2 mice. (H) OCR of splenic and BM early erythroblasts under steady state and serial bleeding stress. (I) Protein synthesis rate, as determined by OP-Puro incorporation, of splenic and BM early erythroblasts under steady state and serial bleeding stress. (J) Protein synthesis rate of splenic and BM early erythroblasts, with or without transferred mitochondria, from serially bled mice. (K) ATP content of splenic and BM early erythroblasts, with or without transferred mitochondria, from serially bled mice. For all quantification, mean ± SEM; *, P < 0.05; **, P < 0.01; ***, P ≤ 0.001 by Student’s t test. (L) Proposed model of EBI macrophage-mediated erythroid recovery involving transfer of mitochondria from EBI macrophages to early erythroblasts, with is associated with an enhanced proliferative profile, particularly in a CD47 marked erythroblast subset. The mitochondria transfer events are possibly regulated by CD47–Sirpα mediated cell–cell interaction. The illustration was created with BioRender.com.