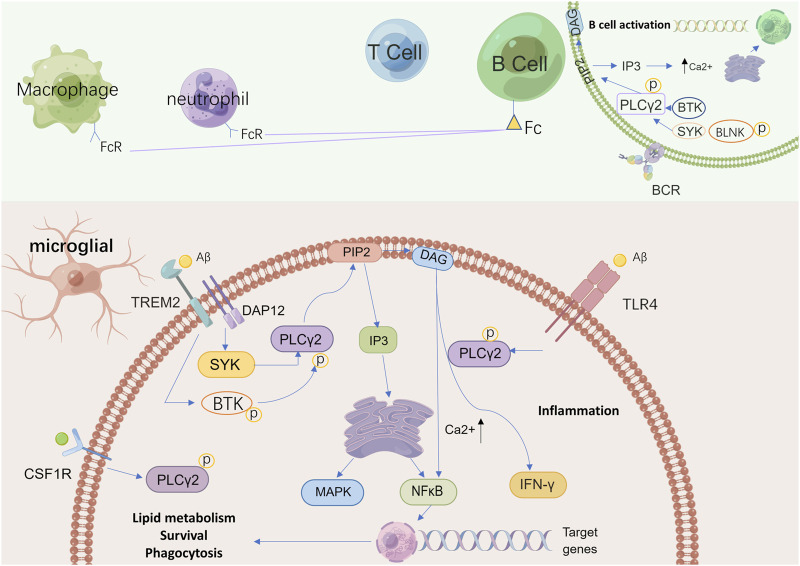
FIGURE 2.
A schematic view of possible pathways related to PLCγ2 in microglia and other immune cells. PLCγ2 belongs to a group of intracellular enzymes that cleave the membrane phospholipid PIP2 to DAG and IP3, resulting in increased calcium signaling. In absence of activating signals, PLCγ2 is neither recruited to the membrane or activated but instead maintained in its autoinhibited form in the cytoplasm. In the brain, PLCγ2 is predominantly expressed by microglia. In microglia, PLCγ2 can be activated downstream of TREM2–DAP12 via interaction with SYK to promote beneficial microglial functionality, while also signaling downstream of TLR4 signaling upon ligand stimulation to induce an inflammatory response. The activation of PLCγ2 finally supports lipid metabolism, phagocytosis, and survival. PLCγ2 activation also leads to BCR signaling, including activation of SYK, BTK, and BLNK with increased calcium signaling. This process affects B cell development and increases the production of neutrophils and macrophages. In these immune cells, as a downstream protein in Fc receptor signaling, PLCγ2 regulates calcium-dependent functions to support immune and inflammatory responses. PIP2: phosphatidylinositol-4,5-bisphosphate; DAG: diacylglycerol; IP3: inositol 1,4,5 trisphosphate; TREM2: triggering receptor expressed on myeloid cells two; DAP12: DNAX-activating protein of 12 kDa; SYK: spleen tyrosine kinase; TLR4: toll-like receptor four; PLCγ2: phospholipase C gamma two; BCR: B cell receptor; BTK: Bruton’s tyrosine kinase; BLNK: B cell linker protein.