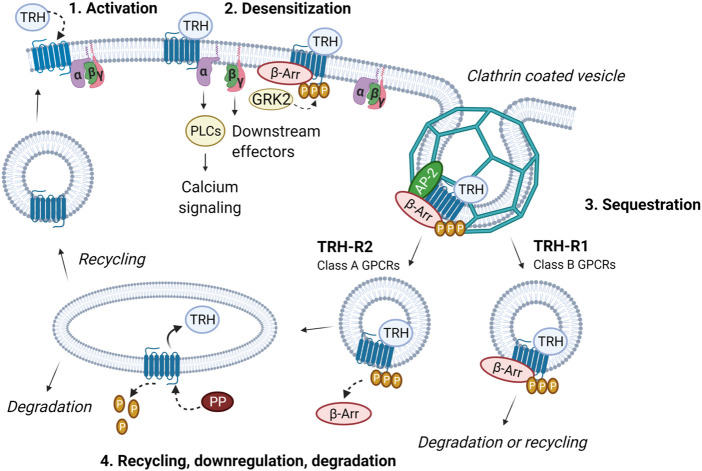
FIGURE 2.
Intracellular trafficking of TRH receptor subtypes. The activation of TRH-R with TRH results in dissociation of Gα and Gβγ and an increase in intracellular calcium. Receptor is then desensitized through phosphorylation by GRK2 and β-arrestin binding. In this way, coupling between the receptor, G protein and effector is abolished and signaling terminated. β-Arrestin functions as an adaptor protein which recruits proteins from the endocytic machinery (i.e., clathrin, AP-2 and others). Subsequently, receptor is sequestered via clathrin-coated vesicles. Internalized TRH-R can then undergo two different processes depending on the receptor subtype and interaction with β-arrestin. TRH-R2, belonging to class A receptors, dissociates from β-arrestin immediately upon internalization. TRH is removed and receptor is dephosphorylated by protein phosphatase (PP). TRH-R2 is then recycled and targeted to the plasma membrane. On the other hand, TRH-R1 which belongs to the class B receptors, forms stable complexes with β-arrestin in endocytic vesicles and can either be degraded or slowly recycled to the plasma membrane. The figure was created by BioRender.