Highlights
-
•
Cell signal transduction is a fundamental process in the development and progression of cancer.
-
•
The mitogen-activated protein kinase (MAPK) pathway comprises several key signaling components and phosphorylation events that play crucial roles in tumorigenesis.
-
•
Phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway is important for tumor cell growth and survival, and often contributes to oncogenesis.
-
•
Aberrant activation of Wnt/β-catenin signaling gives rise to the accumulation of β-catenin in the nucleus and promotes the transcription of many oncogenes and contributes to carcinogenesis and tumor progression.
-
•
Therapy targeting MAPK, PI3K/AKT/mTOR, and Wnt/β-catenin pathways is promising therapeutic strategy.
Keywords: Signaling pathways, MAPK, PI3K/AKT/mTOR, Wnt/β-catenin, Therapeutic opportunities
Abstract
Several different signaling pathways and molecular mechanisms have been identified as responsible for controlling critical functions in human cancer cells, such as selective growth and proliferative advantage, altered stress response favoring overall survival, vascularization, invasion and metastasis, metabolic rewiring, an abetting microenvironment, and immune modulation. This concise summary will provide a selective review of recent studies of key signal transduction pathways, including mitogen-activated protein kinase (MAPK) pathway, Phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling, and Wnt/β-catenin signaling pathway, which are altered in cancer cells, as the novel and promising therapeutic targets.
Introduction
It is well accepted that cancer is driven by successive genetic and epigenetic alterations that allow cells to escape homeostatic controls that ordinarily suppress inappropriate proliferation and inhibit the survival of aberrantly proliferating cells outside their normal niches.
In the development and progression of cancer, cell signal transduction is a fundamental process. Tumor cells possess a set of characteristics or hallmarks, including uncontrolled proliferation, genomic instability, and apoptosis evasion. The intricacy of cellular signaling networks has significant implications for our understanding of tumor cell behavior and our ability to use this knowledge for cancer therapy. Modifications to various cell signaling pathways promote tumor cell proliferation, progression, and survival. The changes in cancer cells result from multiple alterations in cellular signaling machinery. Dysregulation of the pathway is associated with a plethora of human cancers, and there are numerous efforts to target critical components of the pathway for disease intervention. Its intervention that might cause dysregulation relevant for human diseases has been dissected in several signaling pathways. Targeted therapies aim at controlling the signaling pathways through which cell survival and death are regulated.
Here, in order to understand the architecture of the key signaling network, we outline the current general aspects of three key signaling pathways, including the MAPK pathway, PI3K/AKT/mTOR signaling, and Wnt/β-catenin signaling pathway, the components in signaling pathways, and the relationship between factors and therapeutic opportunities.
Key signal transduction pathways
MAPK pathway
The MAPK pathway plays a vital role in signal transduction as a bridge in the switch from extracellular signals to a wide range of cellular responses. MAPK signaling is active in both early and advanced stages of tumorigenesis, and it promotes tumor proliferation, survival, and metastasis. Dysregulation of the MAPK cascade involves key signaling components and phosphorylation events that play an important role in tumorigenesis. MAPK regulatory effects on the cellular constituent of the tumor microenvironment are for immunosuppressive purposes. Cross-talking between MAPK with oncogenic signaling pathways including WNT, cyclooxygenase-2, transforming growth factor-β, NOTCH and (in particular) with phosphatidylinositol 3-kinase is contributed to the multiplication of tumor progression and drug resistance.
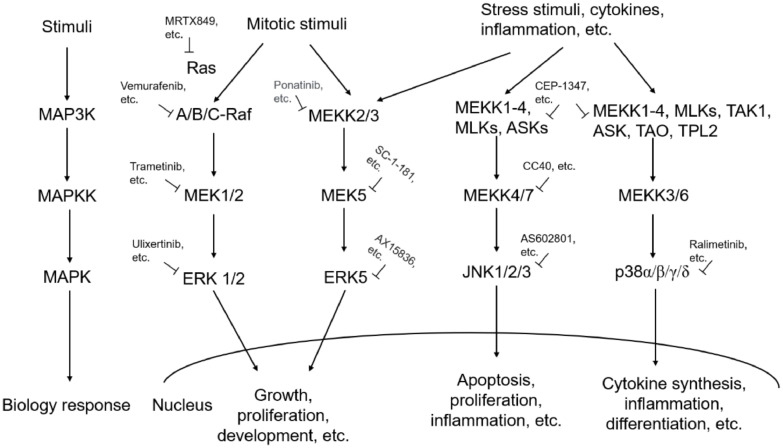
There are four classical MAPK signaling cascades, including the extracellular signal-regulated kinase (ERK)1/2, the c-Jun N-terminal kinase (JNK), the p38, and the ERK5 [1] (Fig. 1). These sub-pathways correspond to the ERK1/2, JNK1/2/3, p38α/β/γ/δ, and ERK5 subfamilies of MAPK (Fig. 1), respectively, and are a chain of proteins that subsequently transmit the signal through a sequence of various extracellular signals to the nucleus, controlling gene expression through transcriptional factors [2]. By regulating protein activities through signaling cascades that consist of MAPK kinase(MAP3K), MAPK kinase (MAPKK) and MAPK and these three central layers are considered as a basic core unit [3].The stress-activated JNKs and p38 MAPKs play key roles in balancing cell survival and death in response to both extracellular and intracellular stresses [4], while the ERK/MAPK signaling pathway, which is the most thoroughly studied MAPK signaling pathway, is closely related to cell proliferation and differentiation and plays a pivotal role in the cell signal transduction network [5]. The activity of the ERK signaling pathway is tightly regulated through the core pathway components. Ras acts as an upstream activating protein, Raf acts as MAP3K, MAPK/ERK kinase (MEK) acts as MAPKK and ERK is the MAPK, forming the Ras-Raf-MEK-ERK pathway [6]. These pathways are considered potential therapeutic targets for cancer treatment.
Fig. 1.
A schematic representation of MAPK pathways, organized into main signaling modules (ERK1/2, JNK, p38 and ERK5), and inhibitors.
The constitutive activation of the Ras/Raf/MAPK pathway was observed in various types of cancers. It has been found that correlation of L antigen family member 3 (LAGE3) with unfavorable prognosis and promoting tumor development in HCC via PI3K/AKT/mTOR and Ras/RAF/MAPK pathways [7]. Furthermore, knockdown of phospholipase C β2 (PLCB2) expression was reported to reduce melanoma cell viability and promotes melanoma cell apoptosis by altering Ras/Raf/MAPK signals [8]. In another study, melatonin has been observed to inhibit the survival of human gastric cancer cells under endoplasmic reticulum (ER) stress involving autophagy and Ras/Raf/MAPK signaling [9]. Additionally, it has been reported that metapristone can suppress non-small cell lung cancer proliferation and metastasis via modulating Ras/Raf/MEK/MAPK signaling pathway [10]. As Ras/Raf/MAPK signaling is complex, there are many steps at which to target therapies designed to interfere with signaling.
Recently, several small molecule inhibitors (SMIs), including MEK1/2 and Raf inhibitors, targeting this pathway have been developed and are currently being tested in clinical evaluation such as CI-1040, PD-0,325,901, ARRY-438,162, AZD6244, RDEA119/BAY 86–9766, GSK1120212, and so on [11,12]. However, targeting these has proven difficult. Since these inhibitors are not able to hit specific target proteins, Ras inhibition did not achieve the expected results in clinical trials. Since approaches to directly target Ras have not been successful, inhibition of Ras remains an interesting target although challenging [11].
A wealth of studies has highlighted the importance of microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) in cancer development. This is likely due to these regulatory RNA elements typically regulate expression of downstream gene expression. The effects of miRNA and lncRNA on MAPK signaling pathway have been found to be altered in many types of cancers. MiRNA-95/MAPK pathway/dual-specificity phosphatase 5 (DUSP5) interaction has been found to regulate epithelial–mesenchymal transition (EMT) and cancer stem cell phenotype in gastric cancer cells [13]. In addition to miR-95, miR-188–5p promotes apoptosis and inhibits cell proliferation of breast cancer cells via the MAPK signaling pathway by targeting Rap2c [14]. MiR-4500 has also been identified to inhibit migration, invasion, and angiogenesis of breast cancer cells via ribonucleotide reductase subunit M2 (RRM2)-dependent MAPK signaling pathway [15]. The relationship between lncRNA and ERK/MAPK signaling pathway has been reported in various types of cancer. LncRNA HEIH enhances paclitaxel-tolerance of endometrial cancer cells via activation of MAPK signaling pathway [16]. Additionally, down-regulation of lncRNA LINC00152 suppresses gastric cancer cell migration and invasion through inhibition of the ERK/MAPK signaling pathway [17]. Given their important roles across tumorigenesis, miRNAs and lncRNAs can be considered as therapeutic targets, since the fine-tuning of their expression can modulate the activity of fundamental pathways in a variety of cancers.
PI3K/AKT/mTOR signaling pathway
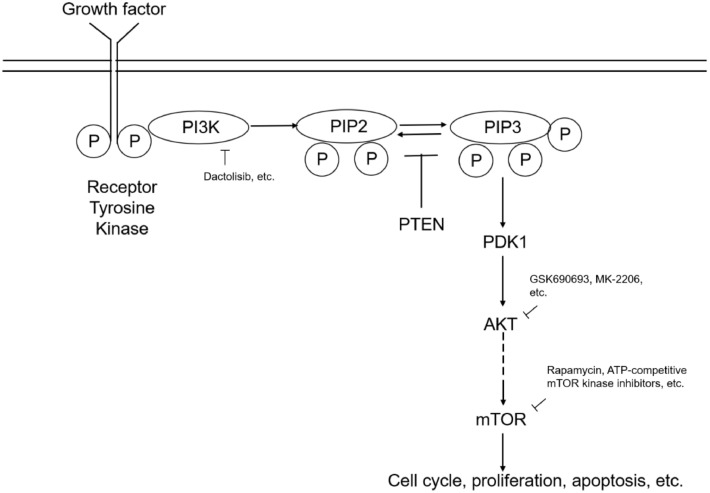
PI3K/AKT/mTOR signaling (Fig. 2) is one of the most important intracellular pathways. Signaling is involved in a variety of cellular functions, such as cell survival, metastasis, and metabolism, and often contributes to oncogenesis and cancer. The PI3K/AKT pathway also plays essential roles in the tumor environment and regulates multiple critical steps in angiogenesis and inflammatory factor recruitment. The PI3K/AKT pathway is also a key regulator of survival during cellular stress. Since tumors exist in an intrinsically stressful environment, such as limited nutrient and oxygen supply and low pH, the role of this pathway in cancer appears to be crucial.
Fig. 2.
A simplified overview of the PI3K/AKT/mTOR pathway and inhibitors.
In order to activate PI3K, the extracellular ligand initially activates the specific receptor. The activated PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) at the 3 positions of the inositol ring to generate phosphatidylinositol (3,4,5)-trisphosphate (PIP3). It recruits two protein kinases to the plasma membrane via their pleckstrin homology interaction domains (pH domains) including AKT (also called protein kinase B, or PKB) and phosphoinositide-dependent protein kinase 1(PDK1). Once recruited to the cell membrane, the AKT is phosphorylated by mTOR complex 2(mTORC2) on Ser473, changing the conformation of the AKT and allowing its phosphorylation on Thr308 by PDK1 [18,19]. The activated AKT phosphorylates target proteins from the cell membrane and lose its connection with the cell membrane. The signal conveys to the cytosol and cell nucleus through phosphorylation of other target proteins there, which ultimately leads to the competitive growth advantage, metastatic competence, angiogenesis, and therapy resistance. Thus, this complex pathway has been reported as one of the most attractive targets for the development of anticancer agents.
Alterations to PI3K/AKT/mTOR pathway have been found in practically all human tumors, including breast cancer [20], hepatocellular carcinoma (HCC) [21], and non-small cell lung cancer (NSCLC) [22], and so on. Since it is one of the most frequently disrupted pathways in malignancies, it becomes a desirable target for treatment. Currently, various types of PI3K-specific inhibitors have been developed. Generally, according to their pharmacokinetic characteristics and capacity to interact with ATP-binding clefts, PI3K inhibitors have been classified into three categories, including pan-PI3K inhibitors, isoform-selective PI3K inhibitors, and dual PI3K/mTOR inhibitors [23]. AKT is also a promising target since it is an effector of the PI3K/AKT/mTOR pathway to activate tumors. AKT activity is controlled in an AKT-dependent manner through phosphorylation and dephosphorylation. AKT inhibitors have been classified into three categories depending on how they impede AKT activity. Depending on how they impede AKT activity, AKT inhibitors are divided into three categories, such as ATP-competitive inhibitors, ATP-competitive inhibitors (GSK690693, ipatasertib, uprosertib, and capivasertib), allosteric inhibitors (MK-2206) and irreversible inhibitors. ATP-competitive inhibitors and allosteric inhibitors have demonstrated the more potent inhibition of AKT in malignant cells [23]. mTOR inhibitors are a type of drug that works by selectively inhibiting mTOR activity. mTOR inhibitors are classified into two categories: rapamycin and its analogs (rapalogs), and ATP-competitive mTOR kinase inhibitors. The former is capable of suppressing mTORC1, and the latter can suppress mTORC1/2 [23]. Dual PI3K/mTOR inhibitors (dactolisib (BEZ235), apitolisib (GDC-0980), gedatolisib (PF-05,212,384), bimiralisib (PQR309), paxalisib (GDC-0084), and voxtalisib (SAR245409, XL765) interact with the ATP-binding cleft of both PI3K and mTOR, reducing the kinase activity of both enzymes and impacting pathway activities more effectively than mTOR kinase inhibitors alone. Dual PI3K/mTOR inhibitors have shown substantial anticancer efficacy in a variety of tumor xenografts.
Although PI3K/mTOR inhibitors have shown some promise in the early-stage trial, further research is needed to establish whether they are more effective than mTOR inhibitors [24], [25], [26], [27].
Recently, it has been well accepted that miRNAs and lncRNAs have emerged as key regulators of a wide range of genes and as the PI3K/AKT/mTOR signaling pathway. Of importance, it has been validated that miRNA can target multiple key components in the PI3K/AKT/mTOR pathway in human tumors. MiR-520a-3p inhibits cell growth and metastasis of NSCLC through PI3K/AKT/mTOR signaling pathway [28]. Another study found that miRNA‑205‑5p functions as a tumor suppressor by negatively regulating VEGFA and PI3K/AKT/mTOR signaling in renal carcinoma cells [29]. For lncRNAs, a previous study demonstrated that knockdown of lncRNA‑HOTAIR downregulates the drug‑resistance of breast cancer cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway [30]. In recent years, the regulatory relationship of lncRNA-miRNAs is currently a research hotspot. Recently, it has been reported that the lncRNA NORAD/miR-520a-3p facilitates malignancy in non-small cell lung cancer via PI3k/AKT/mTOR signaling pathway [31].
Besides, many of these PI3K/AKT/mTOR signaling pathway regulatory small RNAs are associated with clinicopathological features and clinical prognosis cancer, which may provide a potential future application in the diagnosis and therapy of cancers.
Wnt/β-catenin signaling pathway
In 1982, the Wnt gene was first identified from mouse tumor cells, which is synonymous with the Drosophila segment polarity gene Wingless and the murine proto-oncogene integration 1 [32]. Wnt ligands are a large family of 19 secreted glycoproteins produced in the ER. The cell surface receptors transfer signals from the extracellular environment to the cell [33]. The Wnt signaling pathway, is an evolutionarily conserved signaling axis participating in diverse physiological processes such as proliferation, differentiation, apoptosis, migration, invasion and tissue homeostasis [34], [35], [36]. The Wnt signaling pathway consists of two major pathways: the canonical and non-canonical pathways. The canonical pathway is typically referred to as the β-catenin-dependent pathway, whereas the non-canonical pathway does not rely on β-catenin and is responsible for controlling cell movement during morphogenesis [37]. Increasing evidence indicates that dysregulation of the Wnt/β-catenin cascade gives rise to the accumulation of β-catenin in the nucleus and promotes the transcription of many oncogenes such as c-Myc and CyclinD-1. Furthermore, Wnt/β-catenin signaling orchestrates multiple cell signaling cascades, such as epidermal growth factor receptor (EGFR), Hippo/YAP, nuclear factor kappa-B (NF-κB), Notch, Sonic Hedgehog and PI3K/AKT pathway, which contribute to pivotal molecular mechanism in cancer development [38], [39], [40], [41], [42], [43]. As a result, it contributes to carcinogenesis and tumor progression of several cancers including colorectal cancer (CRC) [44], HCC [45], pancreatic cancer (PC) [46], ovarian cancer (OC) [47], and so on. Thus, it will be addressed exclusively hereafter in this review.
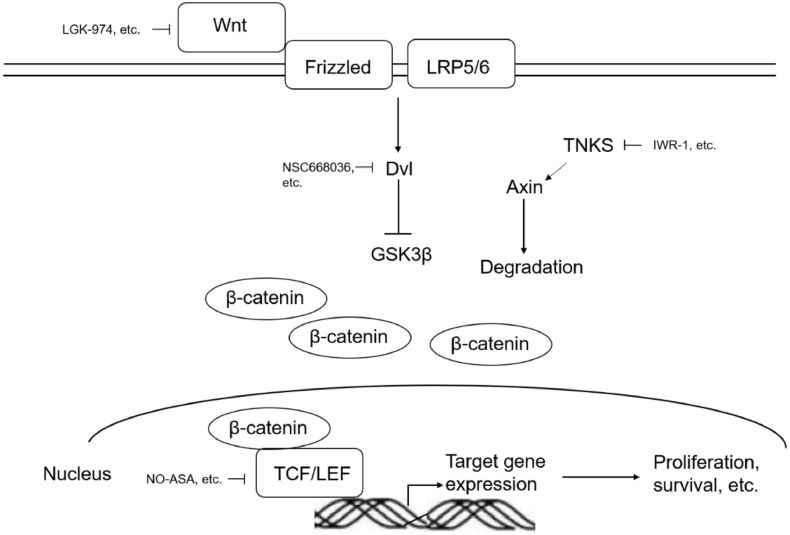
In the on state, a Wnt ligand binds to a seven-pass transmembrane Frizzled (Fz) receptor and its co-receptor, low-density lipoprotein receptor related protein 6 (LRP6) or its close relative LRP5. The formation of a likely Wnt-Fz-LRP6 complex recruits the scaffolding protein Dishevelled (Dvl). After then, low-density lipoprotein receptor-related protein 6 (LRP6) is phosphorylated, activated and recruit the Axin complex to the receptors. These events inhibit Axin-mediated β-catenin phosphorylation and thereby stabilize β-catenin, which accumulates and travels to the nucleus to form complexes with T cell-specific factor (TCF)/lymphoid enhancer-binding factor (LEF) and activates Wnt target gene expression [48]. A schematic representation of the canonical Wnt pathway in its on state was shown in Fig. 3.
Fig. 3.
Schematic representation of the canonical Wnt pathway in its on state and its inhibitors. In the presence of active Wnt, β-catenin accumulates in the cytoplasm, then localizes to the nucleus, and activates transcription together with TCF/LEF transcription factors.
Up to date, since Wnt/β-catenin signaling has been broadly implicated in human cancers and experimental cancer models of animals, increasing investigations highlight the therapeutic potential of agents targeting Wnt/β-catenin signaling in cancer. Many therapies targeting Wnt/β-catenin signaling in cancers have been demonstrated as promising targets. It is assumed new opportunities of developing more satisfactory and precise remedies for cancer patients with aberrant Wnt/β-catenin signaling. However, important challenges are still ongoing when dealing with clinical translations of Wnt/β-catenin signaling-dependent targeted therapies. Currently, the promising preclinical target therapies include SMI targeting Porc, monoclonal antibody (mAb) targeting Wnt receptors and co-receptors, peptide mimetics, SMIs targeting cytoplasmic proteins and agents targeting protein-protein interaction (PPC) in the nucleus [49]. Other than those synthetic compounds, natural products have also been studied and implemented to repress Wnt/β-catenin signaling, including resveratrol [50] and silibinin [51]. Moreover, many FDA-approved drugs have expanded their indications to crosstalk with Wnt/β-catenin signaling, such as pyrvinium [52], niclosamide [53] and salinomycin [54]. However, the functions of these old drugs for targeting Wnt/β-catenin signaling are still limitedly known.
In addition to the above finding, crosstalk between Wnt/β-catenin and other regulatory factors also modulates canonical Wnt signaling in different ways, such as miRNAs and lncRNAs. Some miRNAs enhance the Wnt/β-catenin pathway by inhibiting the negative regulatory factors of the Wnt/β-catenin pathway. For instance, highly expressed miR-92a-3p reduces the ubiquitin-mediated degradation of β-catenin by directly inhibiting F‑box/WD repeat‑containing protein 7 (FBXW7) and modulator of apoptosis protein1 (MOAP1) and promoting the progression of CRC [55]. Down-regulation of miR-31–5p inhibits proliferation and invasion of osteosarcoma cells through Wnt/β-catenin signaling pathway by enhancing AXIN1 [56]. miR‐192 and ‐215 activate Wnt/β‐catenin signaling pathway in gastric cancer (GC) via adenomatous polyposis coli (APC) [57]. Rotavirus-mediated suppression of miR-192 family and miR-181a activates Wnt/β-catenin signaling pathway [58]. On the other hand, some miRNAs play a critical role as negative modulators of the Wnt/β-catenin pathway. MiR-96–5p represses breast cancer proliferation and invasion through Wnt/β-catenin signaling via targeting catenin delta 1 (CTNND1) [59]. MiR-140 represses esophageal cancer progression via targeting zinc finger E-box-binding homeobox 2 (ZEB2) to regulate Wnt/β-catenin pathway [60]. MiR‑769‑3p inhibits tumor progression in glioma by suppressing ZEB2 and inhibiting the Wnt/β‑catenin signaling pathway [61]. LncRNAs also have a remarkably regulatory effect on Wnt/β-catenin, and directly or indirectly modulate the canonical Wnt pathway. LncRNA LINC00673-v4 promotes aggressiveness of lung adenocarcinoma via activating WNT/β-catenin signaling [62]. Hypoxia-induced lncRNA RBM5-AS1 promotes tumorigenesis via activating Wnt/β-catenin signaling in breast cancer [63].
MiR-based therapeutics have been developed based on the regulatory network of miRs and Wnt/β-catenin, such as miR-34 [64]. Several strategies such as miR sponges, anti-miR oligonucleotides, miR masks, and small molecule inhibitors have been used in miR-based anticancer therapeutic approaches. The network of lncRNAs and Wnt/β-catenin signaling exhibits potential for the discovery of novel diagnostics and therapeutics for cancer. A large number of lncRNAs have been identified to be associated with Wnt/β-catenin signaling in many types of cancers through regulating the key factors of Wnt signaling. However, some Wnt pathway-related lncRNAs and their detailed functions and specificity remain unclear. Despite these challenges, targeting miRNAs and lncRNAs in cancer for molecular alterations in cancer genes and associated signaling pathways are used to inform new treatments for precision medicine in cancer.
Future directions
The key regulatory pathways, such as MAPK pathway, PI3K/AKT/mTOR signaling, and Wnt/β-catenin signaling pathway, play essential roles in the regulation of signal transduction and biological processes such as cell proliferation, apoptosis, metabolism, and so on. The regulatory mechanisms and biological functions of these signaling pathways are important in many human cancers. Nevertheless, although the combination of several therapeutic approaches has been promising, many challenges remain to be solved in order to design an effective anti-tumoral treatment that targets tumoral cells. More importantly, such research can provide new molecular targets, biological markers, and genetic diagnoses, for the diagnosis and treatment of cancers.
Data availability
All the data during the current study are included in the article or uploaded as supplementary information.
CRediT authorship contribution statement
Dongliao Fu: Methodology, Writing – original draft, Writing – review & editing. Zhigang Hu: Writing – original draft, Writing – review & editing. Xinyang Xu: Data curation, Visualization. Xiaoyan Dai: Supervision, Writing – review & editing, Methodology, Funding acquisition, Writing – original draft. Ziyi Liu: Supervision, Writing – review & editing, Methodology, Funding acquisition, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
Funding
This work received no funding.
Ethics approval and consent to participate
The experimental protocols were approved by the Ethics Committee of Basic Medical Sciences Institute of Chinese Academy of Medical Sciences (No. 028–2013). This paper has not been published elsewhere in whole or in part. All authors have read and approved the content, and agree to submit it for consideration for publication in your journal. Informed consent was obtained from all individual participants included in the study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2022.101510.
Contributor Information
Xiaoyan Dai, Email: xdai@gzhmu.edu.cn.
Ziyi Liu, Email: ziyi.liu@nih.gov.
Appendix. Supplementary materials
References
- 1.Burotto M., Chiou V.L., Lee J.M., Kohn E.C. The MAPK pathway across different malignancies: a new perspective. Cancer. 2014;120(22):3446–3456. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang S.H., Sharrocks A.D., Whitmarsh A.J. MAP kinase signalling cascades and transcriptional regulation. Gene. 2013;513(1):1–13. doi: 10.1016/j.gene.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Guo Y.J., Pan W.W., Liu S.B., Shen Z.F., Xu Y., Hu L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020;19(3):1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner E.F., Nebreda A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer. 2009;9(8):537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 5.Samatar A.A., Poulikakos P.I. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat. Rev. Drug Discov. 2014;13(12):928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 6.Yang S., Liu G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol. Lett. 2017;13(3):1041–1047. doi: 10.3892/ol.2017.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Xiong H. Correlation of LAGE3 with unfavorable prognosis and promoting tumor development in HCC via PI3K/AKT/mTOR and Ras/RAF/MAPK pathways. BMC Cancer. 2022;22(1):298. doi: 10.1186/s12885-022-09398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H., Xie T., Shui Y., Qi Y. Knockdown of PLCB2 expression reduces melanoma cell viability and promotes melanoma cell apoptosis by altering Ras/Raf/MAPK signals. Mol. Med. Rep. 2020;21(1):420–428. doi: 10.3892/mmr.2019.10798. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y., Yuan K., Tang M., Yue J., Bao L., Wu S., Zhang Y., Li Y., Wang Y., Ou X., Gou J., Zhao Q., Yuan L. Melatonin inhibiting the survival of human gastric cancer cells under ER stress involving autophagy and Ras-Raf-MAPK signalling. J. Cell. Mol. Med. 2021;25(3):1480–1492. doi: 10.1111/jcmm.16237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng G., Shen Z., Chen H., Liu J., Jiang K., Fan L., Jia L., Shao J. Metapristone suppresses non-small cell lung cancer proliferation and metastasis via modulating RAS/RAF/MEK/MAPK signaling pathway. Biomed. Pharmacother. 2017;90:437–445. doi: 10.1016/j.biopha.2017.03.091. [DOI] [PubMed] [Google Scholar]
- 11.Santarpia L., Lippman S.M., El-Naggar A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets. 2012;16(1):103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu M., Wang Y., Zhan X. The MAPK pathway-based drug therapeutic targets in pituitary adenomas. Front. Endocrinol. 2019;10:330. doi: 10.3389/fendo.2019.00330. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du M., Zhuang Y., Tan P., Yu Z., Zhang X., Wang A. microRNA-95 knockdown inhibits epithelial-mesenchymal transition and cancer stem cell phenotype in gastric cancer cells through MAPK pathway by upregulating DUSP5. J. Cell. Physiol. 2020;235(2):944–956. doi: 10.1002/jcp.29010. [DOI] [PubMed] [Google Scholar]
- 14.Zhu X., Qiu J., Zhang T., Yang Y., Guo S., Li T., Jiang K., Zahoor A., Deng G., Qiu C. MicroRNA-188-5p promotes apoptosis and inhibits cell proliferation of breast cancer cells via the MAPK signaling pathway by targeting Rap2c. J. Cell. Physiol. 2020;235(3):2389–2402. doi: 10.1002/jcp.29144. [DOI] [PubMed] [Google Scholar]
- 15.Li S., Mai H., Zhu Y., Li G., Sun J., Li G., Liang B., Chen S. MicroRNA-4500 inhibits migration, invasion, and angiogenesis of breast cancer cells via RRM2-dependent MAPK signaling pathway. Mol. Ther. Nucl. Acids. 2020;21:278–289. doi: 10.1016/j.omtn.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo J.L., Tang T., Li J.H., Yang Y.H., Zhang L., Quan Y. LncRNA HEIH enhances paclitaxel-tolerance of endometrial cancer cells via activation of MAPK signaling pathway. Pathol. Oncol. Res. 2020;26(3):1757–1766. doi: 10.1007/s12253-019-00718-w. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y., Sun H. Down-regulation of lncRNA LINC00152 suppresses gastric cancer cell migration and invasion through inhibition of the ERK/MAPK signaling pathway. Oncol. Targets Ther. 2020;13:2115–2124. doi: 10.2147/OTT.S217452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellacosa A., Testa J.R., Moore R., Larue L. A portrait of AKT kinases: human cancer and animal models depict a family with strong individualities. Cancer Biol. Ther. 2004;3(3):268–275. doi: 10.4161/cbt.3.3.703. [DOI] [PubMed] [Google Scholar]
- 19.Manning B.D., Cantley L.C. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong C., Wu J., Chen Y., Nie J., Chen C. Activation of PI3K/AKT/mTOR pathway causes drug resistance in breast cancer. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.628690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Cui X., Hao G., He J. Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma. Open Life Sci. 2021;16(1):1037–1044. doi: 10.1515/biol-2021-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y.J., Wei R.S., Li X.H., Li Q., Yu J.R., Zhuang X.F. MiR-421 promotes lipid metabolism by targeting PTEN via activating PI3K/AKT/mTOR pathway in non-small cell lung cancer. Epigenomics. 2022 doi: 10.2217/epi-2021-0229. [DOI] [PubMed] [Google Scholar]
- 23.Peng Y., Wang Y., Zhou C., Mei W., Zeng C. PI3K/Akt/mTOR pathway and its role in cancer therapeutics: are we making headway? Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.819128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillmann P., Fabbro D. PI3K/mTOR pathway inhibition: opportunities in oncology and rare genetic diseases. Int. J. Mol. Sci. 2019;20(22) doi: 10.3390/ijms20225792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra R., Patel H., Alanazi S., Kilroy M.K., Garrett J.T. PI3K inhibitors in cancer: clinical implications and adverse effects. Int. J. Mol. Sci. 2021;22(7) doi: 10.3390/ijms22073464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanhaesebroeck B., Perry M.W.D., Brown J.R., Andre F., Okkenhaug K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021;20(10):741–769. doi: 10.1038/s41573-021-00209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J., Nie J., Ma X., Wei Y., Peng Y., Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol. Cancer. 2019;18(1):26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lv X., Li C.Y., Han P., Xu X.Y. MicroRNA-520a-3p inhibits cell growth and metastasis of non-small cell lung cancer through PI3K/AKT/mTOR signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22(8):2321–2327. doi: 10.26355/eurrev_201804_14822. [DOI] [PubMed] [Google Scholar]
- 29.Huang J., Wang X., Wen G., Ren Y. miRNA2055p functions as a tumor suppressor by negatively regulating VEGFA and PI3K/Akt/mTOR signaling in renal carcinoma cells. Oncol. Rep. 2019;42(5):1677–1688. doi: 10.3892/or.2019.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z., Qian J., Li J., Zhu C. Knockdown of lncRNA-HOTAIR downregulates the drug-resistance of breast cancer cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2019;18(1):435–442. doi: 10.3892/etm.2019.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan Y., Yao Z., Chen W., Li D. The lncRNA NORAD/miR-520a-3p facilitates malignancy in non-small cell lung cancer via PI3k/Akt/mTOR signaling pathway. Onco Targets Ther. 2020;13:1533–1544. doi: 10.2147/OTT.S230954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 33.Komiya Y., Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi B.R., Cave C., Na C.H., Sockanathan S. GDE2-dependent activation of canonical wnt signaling in neurons regulates oligodendrocyte maturation. Cell Rep. 2020;31(5) doi: 10.1016/j.celrep.2020.107540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salik B., Yi H., Hassan N., Santiappillai N., Vick B., Connerty P., Duly A., Trahair T., Woo A.J., Beck D., Liu T., Spiekermann K., Jeremias I., Wang J., Kavallaris M., Haber M., Norris M.D., Liebermann D.A., D'Andrea R.J., Murriel C., Wang J.Y. Targeting RSPO3-LGR4 signaling for leukemia stem cell eradication in acute myeloid leukemia. Cancer Cell. 2020;38(2):263–278. doi: 10.1016/j.ccell.2020.05.014. e266. [DOI] [PubMed] [Google Scholar]
- 36.Soleas J.P., D'Arcangelo E., Huang L., Karoubi G., Nostro M.C., McGuigan A.P., Waddell T.K. Assembly of lung progenitors into developmentally-inspired geometry drives differentiation via cellular tension. Biomaterials. 2020;254 doi: 10.1016/j.biomaterials.2020.120128. [DOI] [PubMed] [Google Scholar]
- 37.Veeman M.T., Axelrod J.D., Moon R.T. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 2003;5(3):367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 38.Chen L., Qin F., Deng X., Avruch J., Zhou D. Hippo pathway in intestinal homeostasis and tumorigenesis. Protein Cell. 2012;3(4):305–310. doi: 10.1007/s13238-012-2913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu T., Li C. Convergence between Wnt-beta-catenin and EGFR signaling in cancer. Mol. Cancer. 2010;9:236. doi: 10.1186/1476-4598-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnamurthy N., Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat. Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu T., Wei Q., Jin J., Luo Q., Liu Y., Yang Y., Cheng C., Li L., Pi J., Si Y., Xiao H., Li L., Rao S., Wang F., Yu J., Yu J., Zou D., Yi P. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids. Res. 2020;48(7):3816–3831. doi: 10.1093/nar/gkaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry J.M., Tao F., Roy A., Lin T., He X.C., Chen S., Lu X., Nemechek J., Ruan L., Yu X., Dukes D., Moran A., Pace J., Schroeder K., Zhao M., Venkatraman A., Qian P., Li Z., Hembree M., Paulson A., He Z., Xu D., Tran T.H., Deshmukh P., Nguyen C.T., Kasi R.M., Ryan R., Broward M., Ding S., Guest E., August K., Gamis A.S., Godwin A., Sittampalam G.S., Weir S.J., Li L. Overcoming Wnt-beta-catenin dependent anticancer therapy resistance in leukaemia stem cells. Nat. Cell Biol. 2020;22(6):689–700. doi: 10.1038/s41556-020-0507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomar V.S., Patil V., Somasundaram K. Temozolomide induces activation of Wnt/beta-catenin signaling in glioma cells via PI3K/Akt pathway: implications in glioma therapy. Cell Biol. Toxicol. 2020;36(3):273–278. doi: 10.1007/s10565-019-09502-7. [DOI] [PubMed] [Google Scholar]
- 44.Sun J., Zhang T., Cheng M., Hong L., Zhang C., Xie M., Sun P., Fan R., Wang Z., Wang L., Zhong J. TRIM29 facilitates the epithelial-to-mesenchymal transition and the progression of colorectal cancer via the activation of the Wnt/beta-catenin signaling pathway. J. Exp. Clin. Cancer Res. 2019;38(1):104. doi: 10.1186/s13046-019-1098-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Yang B., Wang S., Xie H., Wang C., Gao X., Rong Y., Liu Z., Lu Y. KIF18B promotes hepatocellular carcinoma progression through activating Wnt/beta-catenin-signaling pathway. J. Cell. Physiol. 2020;235(10):6507–6514. doi: 10.1002/jcp.29444. [DOI] [PubMed] [Google Scholar]
- 46.Xu D., Yuan H., Meng Z., Yang C., Li Z., Li M., Zhang Z., Gan Y., Tu H. Cadherin 13 inhibits pancreatic cancer progression and epithelial-mesenchymal transition by Wnt/beta-catenin signaling. J. Cancer. 2020;11(8):2101–2112. doi: 10.7150/jca.37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y., Jin Z., Wang C. Glycogen phosphorylase B promotes ovarian cancer progression via Wnt/beta-catenin signaling and is regulated by miR-133a-3p. Biomed. Pharmacother. 2019;120 doi: 10.1016/j.biopha.2019.109449. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu F., Yu C., Li F., Zuo Y., Wang Y., Yao L., Wu C., Wang C., Ye L. Wnt/beta-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther. 2021;6(1):307. doi: 10.1038/s41392-021-00701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashrafizadeh M., Ahmadi Z., Farkhondeh T., Samarghandian S. Resveratrol targeting the Wnt signaling pathway: a focus on therapeutic activities. J. Cell. Physiol. 2020;235(5):4135–4145. doi: 10.1002/jcp.29327. [DOI] [PubMed] [Google Scholar]
- 51.Villota H., Rothlisberger S., Pedroza-Diaz J. Modulation of the canonical Wnt signaling pathway by dietary polyphenols, an opportunity for colorectal cancer chemoprevention and treatment. Nutr. Cancer. 2022;74(2):384–404. doi: 10.1080/01635581.2021.1884730. [DOI] [PubMed] [Google Scholar]
- 52.Thorne C.A., Hanson A.J., Schneider J., Tahinci E., Orton D., Cselenyi C.S., Jernigan K.K., Meyers K.C., Hang B.I., Waterson A.G., Kim K., Melancon B., Ghidu V.P., Sulikowski G.A., LaFleur B., Salic A., Lee L.A., Miller D.M. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat. Chem. Biol. 2010;6(11):829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egashira I., Takahashi-Yanaga F., Nishida R., Arioka M., Igawa K., Tomooka K., Nakatsu Y., Tsuzuki T., Nakabeppu Y., Kitazono T., Sasaguri T. Celecoxib and 2,5-dimethylcelecoxib inhibit intestinal cancer growth by suppressing the Wnt/beta-catenin signaling pathway. Cancer Sci. 2017;108(1):108–115. doi: 10.1111/cas.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu W., Li Y. Salinomycin suppresses LRP6 expression and inhibits both Wnt/beta-catenin and mTORC1 signaling in breast and prostate cancer cells. J. Cell. Biochem. 2014;115(10):1799–1807. doi: 10.1002/jcb.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu J.L., Wang W., Lan X.L., Zeng Z.C., Liang Y.S., Yan Y.R., Song F.Y., Wang F.F., Zhu X.H., Liao W.J., Liao W.T., Ding Y.Q., Liang L. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer. 2019;18(1):91. doi: 10.1186/s12943-019-1019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X., Zhong L., Li X., Liu W., Zhao Y., Li J. Down-regulation of microRNA-31-5p inhibits proliferation and invasion of osteosarcoma cells through Wnt/beta-catenin signaling pathway by enhancing AXIN1. Exp. Mol. Pathol. 2019;108:32–41. doi: 10.1016/j.yexmp.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Deng S., Zhang X., Qin Y., Chen W., Fan H., Feng X., Wang J., Yan R., Zhao Y., Cheng Y., Wei Y., Fan X., Ashktorab H., Smoot D., Meltzer S.J., Li S., Li K., Peng Y., Jin Z. miRNA-192 and -215 activate Wnt/beta-catenin signaling pathway in gastric cancer via APC. J. Cell. Physiol. 2020;235(9):6218–6229. doi: 10.1002/jcp.29550. [DOI] [PubMed] [Google Scholar]
- 58.Banerjee A., Chawla-Sarkar M., Mukherjee A. Rotavirus-mediated suppression of miRNA-192 family and miRNA-181a Activates Wnt/beta-catenin signaling pathway: an in vitro study. Viruses. 2022;14(3) doi: 10.3390/v14030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao X.H., Zhang Y.L., Zhang Z.Y., Guo S.S., Chen X.B., Guo Y.Z. MicroRNA-96-5p represses breast cancer proliferation and invasion through Wnt/beta-catenin signaling via targeting CTNND1. Sci. Rep. 2020;10(1):44. doi: 10.1038/s41598-019-56571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang S., Li X., Shen W., Hu H., Li C., Han G. MicroRNA-140 represses esophageal cancer progression via targeting ZEB2 to regulate Wnt/beta-catenin pathway. J. Surg. Res. 2021;257:267–277. doi: 10.1016/j.jss.2020.07.074. [DOI] [PubMed] [Google Scholar]
- 61.Wang K., Yang S., Gao Y., Zhang C., Sui Q. MicroRNA-769-3p inhibits tumor progression in glioma by suppressing ZEB2 and inhibiting the Wnt/beta-catenin signaling pathway. Oncol. Lett. 2020;19(1):992–1000. doi: 10.3892/ol.2019.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guan H., Zhu T., Wu S., Liu S., Liu B., Wu J., Cai J., Zhu X., Zhang X., Zeng M., Li J., Song E., Li M. Long noncoding RNA LINC00673-v4 promotes aggressiveness of lung adenocarcinoma via activating WNT/beta-catenin signaling. Proc. Natl. Acad. Sci. U. S. A. 2019;116(28):14019–14028. doi: 10.1073/pnas.1900997116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X., Yang J., Ni R., Chen J., Zhou Y., Song H., Jin L., Pan Y. Hypoxia-induced lncRNA RBM5-AS1 promotes tumorigenesis via activating Wnt/beta-catenin signaling in breast cancer. Cell Death. Dis. 2022;13(2):95. doi: 10.1038/s41419-022-04536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim N.H., Kim H.S., Kim N.G., Lee I., Choi H.S., Li X.Y., Kang S.E., Cha S.Y., Ryu J.K., Na J.M., Park C., Kim K., Lee S., Gumbiner B.M., Yook J.I., Weiss S.J. p53 and microRNA-34 are suppressors of canonical Wnt signaling. Sci. Signal. 2011;4(197):ra71. doi: 10.1126/scisignal.2001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data during the current study are included in the article or uploaded as supplementary information.