Abstract
Background
Sepsis-associated acute kidney injury (AKI) often worsens with the deterioration of a patient's condition. Therefore, we hypothesized that monitoring AKI dynamically from day 1 to day 3 was potential to predict hospital mortality. Specifically, we explored whether monitoring AKI dynamically in the intensive care unit (ICU) could be a sepsis phenotype predictive of mortality. A new classification was established based on the change in the AKI stage from admission day 1 and day 3. We compared the hospital mortality, cytokines, and immune response pattern between each group.
Methods
We retrospectively enrolled 523 patients with sepsis, and we calculated the AKI stages on day 1 and day 3 admission to ICUs.
Among these 523 people, 388 of them were assigned to normal, improved, and deteriorated groups according to the changes in the AKI stages. 263 of which did not develop AKI on day 1 and day 3 (normal group). The AKI stage improved in 68 patients (improved group) and worsened in 57 (deteriorated group). We compared the mortality rates between the groups, and identified the relationship between the dynamic AKI status, immune response patterns, and cytokine levels.
Results
The hospital mortality rate in the deteriorated group was higher than that in the non-deteriorated group (combination of normal and improved group) (p = 0.004). Additionally, according to the Kaplan–Meier analysis, the non-deteriorated group had a distinct hospital survival curve (p = 0.004). Furthermore, both the overexpression of tumor necrosis factor-α and decreased monocyte expression of human leukocyte antigen-DR were present in the deteriorated group.
Conclusions
The deteriorated group was associated with a higher hospital mortality rate, potentially resulting from an abnormal inflammatory response. Worsening AKI in the first 3 days of ICU admission may be a sepsis phenotype predictive of hospital mortality.
Keywords: Sepsis phenotype, Acute kidney injury, Dynamic acute kidney injury stage, Immune response
At a glance of commentary
Scientific background on the subject
Acute renal injury is a warning sign of poor prognosis in the treatment of sepsis. By observing the deterioration of kidney function, physicians can predict the patient’s outcome earlier and give appropriate treatment individually.
What this study adds to the field
This article brings an idea that observing the changes in kidney function in the first three days could be closer to the clinical results than observing the kidney status on admission only. This article also gives a glance of cytokine changes between patients with normal, improved, or worsen kidney status.
Sepsis is a life-threatening condition resulting from a dysregulated immune response to an infective insult [[1], [2], [3]]. It is the primary reason for intensive care unit (ICU) admissions, and is associated with high mortality and morbidity [4,5], as it often leads to widespread organ injury. Acute kidney injury (AKI) is a frequent complication of sepsis that may synergistically increase mortality rates [[6], [7], [8]].
When treating sepsis, dynamic rather than static variables are used to monitor a patient's response [9]. Dynamic tools perform better than initial static values for risk stratification [10]. We speculated that a simple dynamic renal function evaluation tool could be used to predict outcomes in sepsis. Hence, we established a new classification determined by the change in AKI stage from day 1 to day 3 of admission to the ICU. We hypothesized that our classification would be closely correspond to mortality outcomes.
Although inflammation plays a role in the pathogenesis of AKI, AKI itself has profound immunosuppressive effects [11]. Cytokine levels may be altered during concomitant systemic inflammation. Therefore, a second analysis was performed to identify the cytokine levels and immune response patterns according to our classification. We aimed to determine the correlation between the change in AKI status and cytokine levels and immune response patterns for each subgroup to predict mortality outcomes.
Material and methods
Study design and setting
This was a retrospective study conducted in three medical ICUs (34 beds total) at the Kaohsiung Chang Gung Memorial Hospital, a 2700-bed tertiary teaching hospital in Southern Taiwan. All patients admitted to the ICU between August 2013 and January 2016 were screened for the study. Patients were excluded if they met any of the following criteria: (1) < 18 years of age, (2) no instance of sepsis during admission, and (3) those who died within 3 days of admission. Data from this study population were also analyzed for other specific aims determined beforehand [10,[12], [13], [14], [15], [16], [17], [18]].
Definitions
The criteria used to define sepsis were adopted from the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), which defines sepsis as an increase in the Sequential Organ Failure Assessment (SOFA) score by ≥ 2 points [3]. The AKI stages were stratified based on the 2012 Kidney Disease Improving Global Outcomes Clinical Practice Guidelines for AKI [19], in which the stages are classified as follows: stage 1, an increase in serum creatinine level that is 1.5–1.9 times that taken at baseline or ≥ 0.3 mg/dL; stage 2, an increase in serum creatinine level that is 2.0–2.9 times that taken at baseline; and stage 3, an increase in serum creatinine level that is 3.0 times that taken at baseline or ≥ 4.0 mg/dL or the initiation of renal replacement therapy. The first serum creatinine value measured on admission was considered the baseline level [20].
For patients who lacked serum creatinine data, urine output was used to determine the AKI stage, according to the following criteria: stage 1, urine output <0.5 mL/kg/h for 6–12 h; stage 2, urine output <0.5 mL/kg/h for >12 h; and stage 3, urine output <0.3 mL/kg/h for >24 h or the presence of anuria for >12 h. Chronic kidney disease (CKD) was defined as an abnormality in the structure or function of the kidney present for at least 3 months, with a glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 (GFR categories: G3a–G5) [21]. Most patients in our study had complete creatinine data for both admission days 1 and 3, and the AKI staging was verified by two licensed physicians to reduce the likelihood of misclassification bias.
Categorization
Patients with sepsis were categorized based on changes in renal function and AKI stage from day 1 to day 3 of ICU admission. Patients whose renal function and serum creatinine levels were normal with no AKI on both day 1 and day 3 were classified as the normal group. The improved group was comprised of patients whose AKI stage improved, and the deteriorated group was comprised of patients whose AKI stage worsened.
Immune status and cytokine data
In our prior studies, we investigated immune function and cytokine levels in sepsis [12,16]. We retrospectively reviewed the medical charts and excluded all those with missing data. A total of 74 patients were included in the immune function and cytokine data analysis.
Details regarding the laboratory procedures used have been reported in previous studies. In brief, after plasma and peripheral blood mononuclear cell preparation, the patients’ immune status was determined by measuring the levels of monocyte expression of human leukocyte antigen-DR (HLA-DR) via flow cytometry. In this study, at least 30,000 peripheral blood mononuclear cells per 100 μL of peripheral blood were analyzed from each sample. The results are expressed as the percentage of HLA-DR-positive monocytes within the total monocyte population. We identified immune paralysis through monitoring the downregulation of HLA-DR expression [22].
The pro-inflammatory cytokine levels analyzed included tumor necrosis factor-α (TNF-α), interferon gamma (IFN-γ), and interleukin-6 (IL-6). TNF-α is a well-known pro-inflammatory cytokine. IFN-γ contributes to macrophage and T-cell activation and participates in anti-inflammatory processes such as the induction of anti-inflammatory molecules (e.g., interleukin-1 receptor antagonist [IL-1Ra]) and the modulation of pro-inflammatory cytokine production [23]. IL-6 acts as a pro-inflammatory cytokine with certain anti-inflammatory properties. Circulating IL-6 stimulates the production and release of chemokines, resulting in the recruitment of neutrophils into the uninjured lung [24]. Simultaneously, IL-6 plays a role in interleukin-10 (IL-10) production and limits inflammation of the lungs [25].
IL-10 and IL-1Ra were selected for their anti-inflammatory effects. Both circulating IL-1Ra and IL-10 levels are significantly elevated in sepsis. IL-10 suppresses the production of proinflammatory mediators such as TNF-α, interleukin-1 (IL-1), IL-6, and IFN-γ in immune cells. IL-10 also mitigates AKI-associated lung inflammation [25]. IL-1Ra binds non-productively to the cell surface IL-1 receptor and prevents IL-1 from transmitting a signal to that cell. Therefore, IL-1Ra could potentially be used to predict sepsis mortality [26].
Vascular endothelial growth factor (VEGF) was also selected for its pro-inflammatory effects via cell-mediated immune inflammation and associated angiogenesis. Some studies have demonstrated the renal protective effect of VEGF through associated improvement in renal micro-perfusion [27]. Granulocyte colony-stimulating factor (G-CSF) is an immune modulator that acts on neutrophils to enhance their maturation, survival, and effector function [28]. The levels of selected cytokines were quantified using the MILLIPLEX MAP customized human cytokine/chemokine magnetic bead panel kit.
Statistical analyses
Data analyses were performed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA), and statistical significance was set at a two-sided p value < 0.05. Patient demographics, clinical characteristics, and outcomes are summarized using frequencies and percentages for categorical variables and means ± standard deviations for continuous variables.
We performed a comparative analysis of 7-day, 14-day, 28-day, ICU, and hospital mortality between the groups using the Pearson chi-square test. Inter-group differences were analyzed using one-way analysis of variance followed by a post hoc Bonferroni correction for repeated comparisons. A trend test was performed to examine the relationships between the groups. To analyze the survival curve, we compared time-to-event data using the log-rank test from the Kaplan–Meier survival analysis.
A univariate Cox regression analysis was performed to examine the impact of medical conditions on hospital survival. Several covariates, including mortality predictors (age, Acute Physiology and Chronic Health Evaluation II [APACHE II] score, and body mass index [BMI]) and potential confounders (gender, comorbidities, and progression of AKI stage) were included in the model. Multivariate Cox regression backward elimination (likelihood ratio) models were performed to analyze the association between mortality risk and medical conditions after adjusting for the relevant confounders.
HLA-DR expression (%) is presented in a bar chart, and the distribution of cytokine levels (pg/dL) is shown using a box plot. We performed nonparametric statistics using the Kruskal–Wallis test to detect the statistical significance of each cytokine.
Results
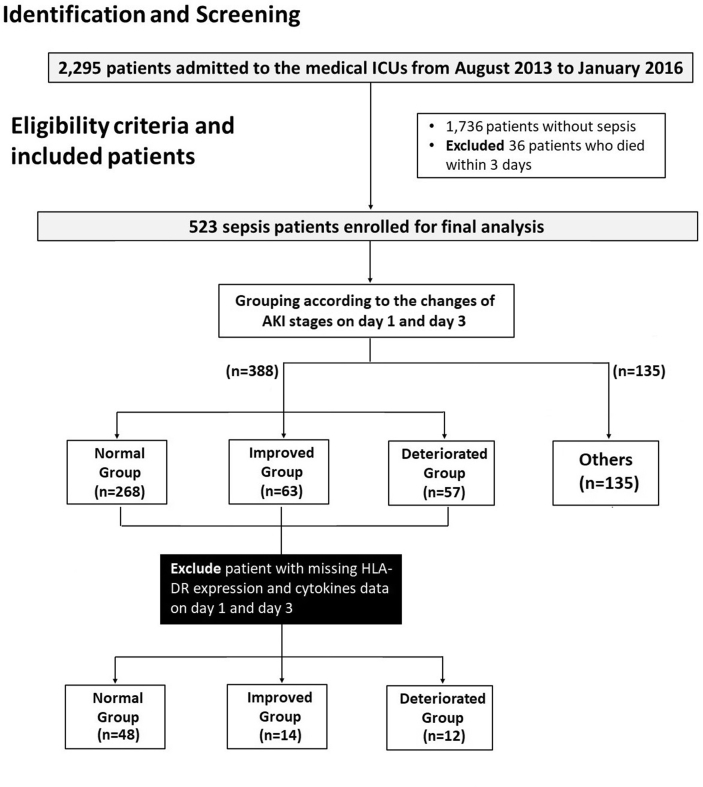
A total of 2295 patients were admitted to the three medical ICUs between August 2013 and January 2016. After excluding patients who did not develop sepsis and those who died within 3 days of admission, 523 patients remained. The new classification was adopted for 388 of these 523 patients. A total of 263 patients were included in the normal group, 68 in the improved group, and 57 in the deteriorated group. After excluding those with missing HLA-DR expression and cytokine level data, a total of 74 patients who had data for both day 1 and day 3 of admission were included (normal group = 48, improved group = 14, deteriorated group = 12) (Fig. 1).
Fig. 1.
Patient selection flowchart. Abbreviations: AKI, acute kidney injury.
The backgrounds and baseline characteristics of the patients are shown in Table 1. The variables, which include primary information (age, sex, and BMI), subscored items from the Charlson comorbidity index, and common possible infectious sources, were collected from the patients’ medical charts.
Table 1.
Characteristics of patients categorized according to the dynamic AKI stage.
| Total (N = 388) | Grouping |
p value | |||
|---|---|---|---|---|---|
| Normal Group (n = 263) | Improved Group (n = 68) | Deteriorated Group (n = 57) | |||
| Basic data | |||||
| Age, years | 67.6 ± 15.1 | 66.9 ± 15.7 | 69.1 ± 14.0 | 69.1 ± 13.3 | 0.631 |
| BMI, kg/m2 | 22.1 ± 4.8 | 21.8 ± 4.9 | 22.8 ± 4.3 | 22.4 ± 4.3 | 0.121 |
| Sex, male | 239 (61.6) | 159 (60.5) | 40 (58.8) | 40 (70.2) | 0.347 |
| APACHE II | 23.6 ± 8.1 | 22.7 ± 8.0 | 25.8 ± 7.3 | 25.0 ± 8.3 | 0.020 |
| SOFA scores | 8.2 ± 3.6 | 7.1 ± 3.1 | 10.7 ± 2.9 | 10.1 ± 4.0 | <0.001 |
| Charlson comorbidity index | 2.7 ± 2.0 | 2.6 ± 2.0 | 2.5 ± 2.0 | 2.8 ± 1.9 | 0.519 |
| Coronary artery disease | 92 (23.7) | 58 (22.1) | 18 (26.5) | 17 (29.8) | 0.359 |
| History of stroke | 110 (21.0) | 57 (21.7) | 15 (22.1) | 13 (22.8) | 0.982 |
| Hypertension | 200 (51.7) | 126 (47.9) | 40 (58.8) | 34 (60.7) | 0.095 |
| COPD | 62 (16.0) | 49 (18.6) | 5 (7.4) | 8 (14.0) | 0.073 |
| Cancer | 98 (25.3) | 64 (24.3) | 16 (23.9) | 18 (31.6) | 0.503 |
| Liver cirrhosis | 35 (9.0) | 24 (9.1) | 5 (7.4) | 6 (10.5) | 0.831 |
| Diabetes mellitus | 160 (41.2) | 104 (39.5) | 31 (45.6) | 26 (45.6) | 0.490 |
| CKD | 59 (15.2) | 0 (0) | 33 (48.5) | 19 (33.3) | 0.009 |
| CKD stage before ICU admission | |||||
| G1-G2 | 367 (70.2) | 263 (100) | 35 (51.5) | 38 (66.7) | |
| G3a | 27 (5.2) | 0 (0) | 9 (13.2) | 10 (17.5) | |
| G3b | 31 (5.9) | 0 (0) | 16 (23.5) | 5 (8.8) | |
| G4 | 31 (5.9) | 0 (0) | 6 (8.8) | 4 (7.0) | |
| G5 | 67 (12.8) | 0 (0) | 2 (2.9) | 0 (0) | |
| Infection sources | |||||
| Lungs | 256 (66.0) | 185 (70.3) | 38 (55.9) | 33 (57.9) | 0.031 |
| UTI | 99 (25.5) | 63 (24.0) | 23 (33.8) | 13 (22.8) | 0.220 |
| Bacteremia | 31 (8.0) | 17 (6.5) | 10 (14.7) | 4 (7.0) | 0.085 |
| Length of stay (LOS) | |||||
| LOS in ICU (days) | 13.4 ± 9.8 | 12.8 ± 8.9 | 15.6 ± 12.5 | 13.0 ± 9. | 0.256 |
| LOS in hospital (days) | 31.0 ± 24.4 | 31.4 ± 24.9 | 33.0 ± 23.3 | 26.3 ± 23.0 | 0.071 |
| Organ support | |||||
| Mechanical ventilation | 487 (92.5) | 245 (93.2) | 64 (94.1) | 50 (87.7) | 0.315 |
| Ventilation days | 22.67 ± 60.0 | 21.4 ± 50.8 | 34.1 ± 101.2 | 14.9 ± 15.3 | 0.408 |
| Vasopressor therapy | 107 (27.6) | 54 (20.5) | 29 (42.6) | 24 (42.1) | <0.001 |
| Receiving HD | 15 (3.9) | 2 (0.8) | 4 (5.9) | 9 (15.8) | <0.001 |
| Temporary HD | 12 (3.1) | 2 (0.8) | 2 (2.9) | 8 (14.0) | <0.001 |
| HD after discharge | 3 (0.8) | 0 (0.0) | 2 (2.9) | 1 (1.8) | 0.033 |
1.Data expressed as n (%) for categorical variables and mean ± standard deviation for continuous variables.
2. Abbreviations: AKI: acute kidney injury; BMI: body mass index; APACHE II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; G2: eGFR ≥60 mL/min/1.73 m2; G3a: eGFR 45–59 mL/min/1.73 m2; G3b: eGFR 30–44 mL/min/1.73 m2; G4: eGFR 15–29 mL/min/1.73 m2; G5: eGFR <15 mL/min/1.73 m2; ICU: intensive care unit; UTI: urinary tract infection; HD: hemodialysis.
Determining whether dynamic monitoring of the AKI stage can be used to stratify patients’ hospital mortality
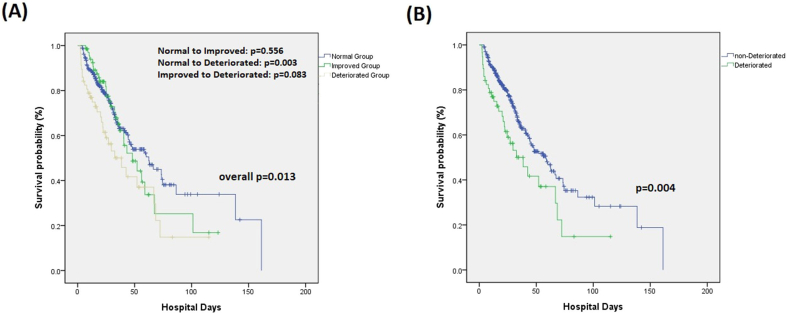
Significant differences in hospital mortality (p = 0.027) and all other mortality outcomes were found between the deteriorated and non-deteriorated groups (Table 2). The Kaplan–Meier analysis also revealed a significant difference in hospital mortality between the deteriorated and non-deteriorated groups (p = 0.004) (Fig. 2A).
Table 2.
Mortality outcomes of patients with sepsis.
| Mortality analysis | Total (N = 388) | Grouping |
p value | p value for trend test | ||
|---|---|---|---|---|---|---|
| Normal Group (n = 263) | Improved Group (n = 68) | Deteriorated Group (n = 57) |
||||
| 7-day mortality | 28 (7.2) | 17 (6.5) | 1 (1.5) | 10 (17.5) | 0.003 | 0.012 |
| 14-day mortality | 49 (12.6) | 32 (12.2) | 4 (5.9) | 13 (22.8) | 0.018 | 0.070 |
| 28-day mortality | 88 (22.7) | 55 (20.9) | 13 (19.1) | 20 (35.1) | 0.053 | 0.034 |
| ICU mortality | 78 (20.1) | 45 (17.1) | 14 (20.6) | 19 (33.3) | 0.021 | 0.007 |
| Hospital mortality |
150 (38.7) |
92 (35.0) |
28 (41.2) |
30 (52.6) |
0.041 |
0.012 |
| Mortality analysis |
Total (N = 388) |
Non-Deteriorated Group (n = 331) |
Deteriorated Group (n = 57) |
p value |
||
| 7-day mortality | 28 (7.2) | 18 (5.4) | 10 (17.5) | 0.003 | ||
| 14-day mortality | 49 (12.6) | 36 (10.9) | 13 (22.8) | 0.017 | ||
| 28-day mortality | 88 (22.7) | 68 (20.5) | 20 (35.1) | 0.018 | ||
| ICU mortality | 78 (20.1) | 59 (17.8) | 19 (33.3) | 0.009 | ||
| Hospital mortality | 150 (38.7) | 120 (36.3) | 30 (52.6) | 0.027 | ||
1Data expressed as n (%) for categorical variables.
2. Abbreviations: ICU: intensive care unit; AKI: acute kidney injury.
Fig. 2.
Hospital survival curve for patients in each categorized group.
Both univariate and multivariate Cox regression analysis were performed to analyze the association between the mortality and prognostic variables. Inclusion in the deteriorated group (HR: 1.702, p = 0.011), a history of stroke (HR: 0.671, p = 0.072), and cancer (HR: 2.225, p < 0.001) were the final independent and significant prognostic variables for hospital mortality after multivariate Cox regression analysis (Table 3).
Table 3.
Univariate and multivariate Cox regression analyses of the predictors of hospital mortality.
| Variable | Univariate Cox regression analysis |
Multivariate Cox regression analysis (likelihood ratio) |
|||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value | ||
| Hospital Mortality |
Age >65 | 1.074 | 0.785–1.470 | 0.656 | |||
| Gender, male | 0.882 | 0.660–1.180 | 0.398 | ||||
| APACHE II | 0.994 | 0.978–1.011 | 0.494 | ||||
| BMI | 0.990 | 0.962–1.019 | 0.490 | ||||
| Coronary heart disease | 0.753 | 0.527–1.077 | 0.121 | ||||
| History of stroke | 0.774 | 0.528–1.135 | 0.189 | 0.671 | 0.434–1.036 | 0.072 | |
| Hypertension | 1.117 | 0.810–1.538 | 0.500 | ||||
| COPD | 1.044 | 0.683–1.598 | 0.841 | ||||
| Cancer | 2.032 | 1.511–2.731 | <0.001 | 2.225 | 1.591–3.111 | <0.001 | |
| Liver cirrhosis | 2.218 | 1.434–3.431 | <0.001 | ||||
| Diabetes mellitus | 0.928 | 0.688–1.253 | 0.627 | ||||
| CKD | 0.937 | 0.608–1.442 | 0.766 | ||||
| Deteriorated group (vs. all other groups) | 1.650 | 1.102–2.471 | 0.015 | 1.702 | 1.131–2.560 | 0.011 | |
Abbreviations: APACHE II: Acute Physiology and Chronic Health Evaluation II; BMI: body mass index; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease.
Immune response patterns according to HLA-DR expression
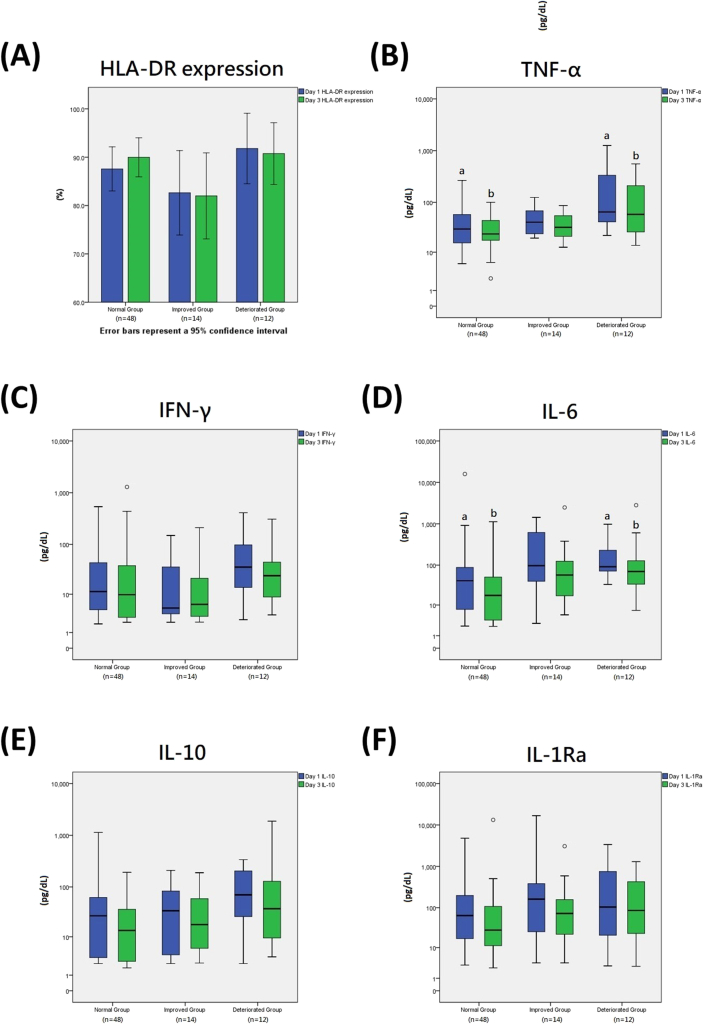
The mean level of HLA-DR expression (%) is presented in a bar chart (Fig. 3A). Mean HLA-DR expression levels were highest in the deteriorated group on the first day, though the mean levels decreased from day 1 to day 3 in both the improved and deteriorated groups.
Fig. 3.
HLA-DR expression and cytokine levels on day 1 and day 3. 1. Error bars in Fig. 3A represent the 95% confidence intervals; outliers in Fig. 3B–F are presented by the white circles above or below the box graphs. 2. The lowercase letters above or below the box graphs in the box plots in Figure 3B–F represent a significant difference (p-value < 0.05) between the two groups that are labeled with the same lowercase letters. 3. Abbreviations: HLA-DR, human leukocyte antigen-DR; TNF-α, tumor necrosis factor-α; IL, interleukin; IFN-γ, interferon-gamma.
Expression of pro-inflammatory cytokines
The distribution of the cytokine levels is presented as a box plot (pg/dL) (Fig. 3B–F). The deteriorated group had the highest median TNF-α levels on day 1 (Fig. 3B) and the highest upper quartile and maximum TNF-α levels, which might suggest an overexpression of TNF-α. Additionally, the TNF-α level in the deteriorated group was significantly higher than that in the normal group on both day 1 (p < 0.001) and day 3 (p = 0.004).
Similarly, the deteriorated group had the highest median and upper quartile IFN-γ levels on both day 1 and day 3 (Fig. 3C). Additionally, the deteriorated group (p = 0.001) had significantly higher levels of IL-6 than the normal group on day 1 (Fig. 3D), and on day 3 (p = 0.002).
Expression of anti-inflammatory cytokines
The deteriorated group had the highest median IL-10 on both day 1 and day 3; however, the differences were not significant for either day (Fig. 3E). Similarly, no significant differences in IL-1Ra levels were found between the groups (Fig. 3F).
Other clinical variables
Essential clinical information associated with sepsis severity is compared in the Appendices. This includes kidney function (BUN, creatinine, fluid intake, urine output, and eGFR), liver function (bilirubin), inflammatory markers (C-reactive protein [CRP], and procalcitonin), and each subitem of the SOFA score. The G-CSF and VEGF levels are also available in the Appendices.
Discussion
AKI is recognized not only as a comorbidity associated with sepsis, but also as a cause of mortality [29]. The development of AKI during sepsis has a significant effect on multiple organ function and is predictive of higher mortality [30]. Serial observations of a patient's response to sepsis treatment is crucial and is associated with a better prediction of mortality than the use of static initial values [10], and our study further confirms this. Significantly different hospital survival curves were observed between the deteriorated and non-deteriorated groups (p = 0.004) (Fig. 2B). In our study, the progression of the AKI stage between days 1 and 3 (deteriorated group) is a potential predictor of hospital mortality (Table 3).
Monitoring dynamic changes in the AKI stage between days 1 and 3 is the cornerstone of our classification, which is easy to calculate and is clinically available information. Compared with the kinetic estimated glomerular filtration rate (KeGFR), initially designed as an easy bedside tool to dynamically assess a patient's AKI status, our categorization is straightforward and can be applied in patients with sepsis. One major assumption of the KeGFR formula is the constant production of creatinine over the course of AKI, which may not be the case for patients with sepsis during fluid resuscitation [31].
Patients with severe chronic comorbidities often have poor outcomes. After adjusting for relevant confounders, our study also revealed other impactful factors, such as a history of cancer (Table 3). This suggests that sepsis outcomes may depend on multiple factors beyond the patient's signs and symptoms. Clinical factors other than kidney condition are also important and should not be dismissed.
The decreased mean level of HLA-DR expression in both the improved and deteriorated groups might reflect HLA-DR downregulation during sepsis. However, the degree of decline was not significant. Therefore, more cases with longer follow-up data regarding HLA expression (e.g., day 7 HLA expression data) are required to determine if HLA-DR expression is delayed in the deteriorated group. The higher TNF-α in the deteriorated group also suggested a hyper-inflammatory status. TNF-α is a powerful pro-inflammatory cytokine released by activated macrophages that activates immune cells and the release of downstream immunoregulatory mediators [32]. The deteriorated group had relatively higher TNF-α levels on the first day, possibly suggesting a more severe inflammatory status in the deteriorated group (Fig. 3B).
A relatively wide distribution of IFN-γ levels were found in the normal group. The upper quartile IFN-γ level on day 1 was higher in the normal group than in the improved group, and the maximum on day 1 in the normal group was higher than that in the deteriorated group (Fig. 3C). This phenomenon might be explained by the complex role that IFN-γ plays in sepsis. IFN-γ is a macrophage and T-cell activating cytokine that also affects immunoregulation. IFN-γ inhibits renal fibrosis and plays a protective role in renal organ-specific autoimmunity [33,34]. Furthermore, IFN-γ has been reported to reverse sepsis-induced immunoparalysis [35]. Improved survival has been shown in patients with sepsis who also have decreased expression of HLA-DR when they are treated with recombinant IFN-γ.
IL-10 acts as an anti-inflammatory cytokine; however, overexpression of IL-10 can be harmful since it can alternatively activate alveolar macrophages and decrease intracellular killing of bacteria, as demonstrated in a mouse model [36]. However, whether the overexpression of IL-10 contributed to the high mortality rate in the deteriorated group in this study is not clear. Although the deteriorated group had higher upper quartile, median, and lower quartile levels of 1L–10 compared to all other groups, there was no statistically significant difference in IL-10 levels between the subgroups. Indeed, the distribution of IL-10 levels varied considerably across the subgroups in our study.
Due to the heterogeneity of sepsis syndrome, correctly selecting the group of patients requiring optimal treatment must be approached carefully [37]. Examining various sepsis phenotypes has potential treatment implications [38]. This phenotype study of sepsis-associated AKI may improve our understanding of the complex pathophysiology of sepsis and help us to determine the appropriate application of these techniques.
A conservative fluid strategy has been suggested as a treatment for patients with sepsis after the initial phase. Fluid balance and immune homeostasis play central roles in the resolution of inflammation [10,39]. Our study demonstrated the positive association between an increase in cumulative fluid balance and mortality. The cumulative fluid balance in the deteriorated group was the highest over the first 72 h. The same phenomenon has also been observed in other studies [[40], [41], [42]].
Additionally, reversal may not be possible with the early or late initiation of renal replacement therapy if the cumulative fluid balance is not affected [43]. Abnormal fluid accumulation may be driven by kidney failure, which can increase mortality by associated malfunction of the heart and lungs. This may suggest the need for a treatment strategy that first focuses on patients at high risk of kidney failure (e.g., dynamic AKI stage-based categorization) and then on an aggressive attempt to return the patient to a euvolemic state. However, this strategy requires validation from further randomized controlled trials.
Our study presented a new strategy of dynamically monitoring the AKI status of patients during their first 3 days of admission, and over-inflammation might explain the poor mortality outcomes in the deteriorated group. The difference in the survival curves between the deteriorated and non-deteriorated groups suggests this classification has the potential to be a useful sepsis phenotyping tool. We made a validation and compared the deteriorated group to non-deteriorated group in all the 523 patients; the survival curve remained distinct (p = 0.034) and the p value became 0.050 if we removed the patients with permanent renal replacement therapy prior to admission (Figure S3-S4).
Our study had some limitations. First, our categorization required AKI status information from day 3; therefore, patients who died before the third day of ICU admission were excluded. Nevertheless, most patients with sepsis admitted to the ICU survived for more than 3 days. Second, the number of patients in the subpopulation with immune profiles was relatively small, especially after they were categorized into subgroups. The likelihood that 74 patients can represent all potential immune responses may be questionable. A little investigation revealed that these 74 patients were relatively evenly distributed across groups (normal group: 48 (26.2%), improved group: 14 (27.9%), and deteriorated group: 12 (26.3%), p = 0.969). Third, since this study was conducted by retrospectively reviewing medical charts, the potential for selection bias must be considered. A multicenter, prospective validation study with clinical variables and immune profiles is warranted in the future.
Conclusions
The dynamic change in AKI status from day 1 to day 3 of ICU admission has the potential to be used as a sepsis phenotype to predict hospital mortality. AKI stage progression is associated with poor mortality outcomes and hyper-inflammatory responses.
Ethics approval and consent to participate
Written informed consent was obtained from the patients or their surrogates in the subpopulation with immune profiles. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital. All procedures were performed in accordance with the relevant guidelines and regulations.
Funding
This work was supported in part by the Chang Gung Memorial Hospital Grant numbers CMRPG8B1063, CMRPG8H1171, CMRPG8J0421, CMRPG8J0422, CMRPG8B1073, CMRPG8B1083, and CMRP8E0731.
Conflicts of interest
The authors have no potential conflicts of interest to declare.
Acknowledgments
We would like to thank all the ICU staff and clinicians who participated in this study for their support. We also wish to thank the Biostatistics Center at Kaohsiung Chang Gung Memorial Hospital for providing statistical consultation.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2021.08.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Singh S., Patra A.K., Patel B., Ramesh G.S., Sharma V.K., Ravishankar V., et al. Acute renal failure in the ICU setting: a prospective observational study. Med J Armed Forces India. 2016;72:236–241. doi: 10.1016/j.mjafi.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang W.F., Chen Y.M., Lin C.Y., Huang H.L., Yeh H., Chang Y.T., et al. Histone deacetylase 2 (HDAC2) attenuates lipopolysaccharide (LPS)-induced inflammation by regulating PAI-1 expression. J Inflamm. 2018;15:3. doi: 10.1186/s12950-018-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international Consensus definitions for sepsis and septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umbro I., Gentile G., Tinti F., Muiesan P., Mitterhofer A.P. Recent advances in pathophysiology and biomarkers of sepsis-induced acute kidney injury. J Infect. 2016;72:131–142. doi: 10.1016/j.jinf.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Gotts J.E., Matthay M.A. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 6.Shum H.P., Kong H.H., Chan K.C., Yan W.W., Chan T.M. Septic acute kidney injury in critically ill patients - a single-center study on its incidence, clinical characteristics, and outcome predictors. Ren Fail. 2016;38:706–716. doi: 10.3109/0886022X.2016.1157749. [DOI] [PubMed] [Google Scholar]
- 7.Uchino S., Kellum J.A., Bellomo R., Doig G.S., Morimatsu H., Morgera S., et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 8.Federspiel C.K., Itenov T.S., Mehta K., Hsu R.K., Bestle M.H., Liu K.D. Duration of acute kidney injury in critically ill patients. Ann Intensive Care. 2018;8:30. doi: 10.1186/s13613-018-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Backer D., Dorman T. Surviving sepsis guidelines: a continuous move toward better care of patients with sepsis. JAMA. 2017;317:807–808. doi: 10.1001/jama.2017.0059. [DOI] [PubMed] [Google Scholar]
- 10.Fang W.F., Huang C.H., Chen Y.M., Hung K.Y., Chang Y.C., Lin C.Y., et al. Application of dynamic pulse pressure and vasopressor tools for predicting outcomes in patients with sepsis in intensive care units. J Crit Care. 2019;52:156–162. doi: 10.1016/j.jcrc.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Singbartl K., Formeck C.L., Kellum J.A. Kidney-immune system crosstalk in AKI. Semin Nephrol. 2019;39:96–106. doi: 10.1016/j.semnephrol.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Fang W.F., Douglas I.S., Chen Y.M., Lin C.Y., Kao H.C., Fang Y.T., et al. Development and validation of immune dysfunction score to predict 28-day mortality of sepsis patients. PloS One. 2017;12 doi: 10.1371/journal.pone.0187088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung K.Y., Chen Y.M., Wang C.C., Wang Y.H., Lin C.Y., Chang Y.T., et al. Insufficient nutrition and mortality risk in septic patients admitted to ICU with a focus on immune dysfunction. Nutrients. 2019;11:367. doi: 10.3390/nu11020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang Y.C., Fang Y.T., Chen H.C., Lin C.Y., Chang Y.P., Chen Y.M., et al. Effect of do-not-resuscitate orders on patients with sepsis in the medical intensive care unit: a retrospective, observational and propensity score-matched study in a tertiary referral hospital in Taiwan. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang Y.C., Huang K.T., Chen Y.M., Wang C.C., Wang Y.H., Tseng C.C., et al. Ventilator dependence risk score for the prediction of prolonged mechanical ventilation in patients who survive sepsis/septic Shock with respiratory failure. Sci Rep. 2018;8:5650. doi: 10.1038/s41598-018-24028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang W.F., Chen Y.M., Lin C.Y., Huang K.T., Kao H.C., Fang Y.T., et al. Immune profiles and clinical outcomes between sepsis patients with or without active cancer requiring admission to intensive care units. PloS One. 2017;12 doi: 10.1371/journal.pone.0179749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang W.F., Chen Y.M., Wang Y.H., Huang C.H., Hung K.Y., Fang Y.T., et al. Incorporation of dynamic segmented neutrophil-to-monocyte ratio with leukocyte count for sepsis risk stratification. Sci Rep. 2019;9:19756. doi: 10.1038/s41598-019-56368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang W.F., Fang Y.T., Huang C.H., Chen Y.M., Chang Y.C., Lin C.Y., et al. Risk factors and associated outcomes of ventilator-associated events developed in 28 days among sepsis patients admitted to intensive care unit. Sci Rep. 2020;10:12702. doi: 10.1038/s41598-020-69731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens P.E., Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 20.De Rosa S., Samoni S., Ronco C. Creatinine-based definitions: from baseline creatinine to serum creatinine adjustment in intensive care. Crit Care. 2016;20:69. doi: 10.1186/s13054-016-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapter 1: definition and classification of CKD. Kidney Int Suppl. 2013;3:19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukaszewicz A.C., Grienay M., Resche-Rigon M., Pirracchio R., Faivre V., Boval B., et al. Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med. 2009;37:2746–2752. doi: 10.1097/CCM.0b013e3181ab858a. [DOI] [PubMed] [Google Scholar]
- 23.Muhl H., Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-gamma. Int Immunopharmacol. 2003;3:1247–1255. doi: 10.1016/S1567-5769(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 24.Ahuja N., Andres-Hernando A., Altmann C., Bhargava R., Bacalja J., Webb R.G., et al. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. Am J Physiol Renal Physiol. 2012;303:F864–F872. doi: 10.1152/ajprenal.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andres-Hernando A., Okamura K., Bhargava R., Kiekhaefer C.M., Soranno D., Kirkbride-Romeo L.A., et al. Circulating IL-6 upregulates IL-10 production in splenic CD4(+) T cells and limits acute kidney injury-induced lung inflammation. Kidney Int. 2017;91:1057–1069. doi: 10.1016/j.kint.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Potjo M., Theron A.J., Cockeran R., Sipholi N.N., Steel H.C., Bale T.V., et al. Interleukin-10 and interleukin-1 receptor antagonist distinguish between patients with sepsis and the systemic inflammatory response syndrome (SIRS) Cytokine. 2019;120:227–233. doi: 10.1016/j.cyto.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Bai Y., Zhang Y., Yang S., Wu M., Fang Y., Feng J., et al. Protective effect of vascular endothelial growth factor against cardiopulmonary bypass-associated acute kidney injury in beagles. Exp Ther Med. 2018;15:963–969. doi: 10.3892/etm.2017.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carulli G. Effects of recombinant human granulocyte colony-stimulating factor administration on neutrophil phenotype and functions. Haematologica. 1997;82:606–616. [PubMed] [Google Scholar]
- 29.Kellum J.A., Angus D.C. Patients are dying of acute renal failure. Crit Care Med. 2002;30:2156–2157. doi: 10.1097/00003246-200209000-00041. [DOI] [PubMed] [Google Scholar]
- 30.Zarjou A., Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22:999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 31.Khayat M.I., Deeth J.M., Sosnov J.A. A bedside clinical tool using creatinine kinetics to predict worsening renal injury and early recovery. Clin Kidney J. 2018;12:248–252. doi: 10.1093/ckj/sfy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulte W., Bernhagen J., Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--an updated view. Mediators Inflamm. 2013;2013:165974. doi: 10.1155/2013/165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitching A.R., Turner A.L., Semple T., Li M., Edgtton K.L., Wilson G.R., et al. Experimental autoimmune anti-glomerular basement membrane glomerulonephritis: a protective role for IFN-gamma. J Am Soc Nephrol. 2004;15:1764–1774. doi: 10.1097/01.asn.0000128968.27705.5e. [DOI] [PubMed] [Google Scholar]
- 34.Oldroyd S.D., Thomas G.L., Gabbiani G., El Nahas A.M. Interferon-gamma inhibits experimental renal fibrosis. Kidney Int. 1999;56:2116–2127. doi: 10.1046/j.1523-1755.1999.00775.x. [DOI] [PubMed] [Google Scholar]
- 35.Leentjens J., Kox M., Koch R.M., Preijers F., Joosten L.A., van der Hoeven J.G., et al. Reversal of immunoparalysis in humans in vivo: a double-blind, placebo-controlled, randomized pilot study. Am J Respir Crit Care Med. 2012;186:838–845. doi: 10.1164/rccm.201204-0645OC. [DOI] [PubMed] [Google Scholar]
- 36.Dolgachev V.A., Yu B., Sun L., Shanley T.P., Raghavendran K., Hemmila M.R. Interleukin 10 overexpression alters survival in the setting of gram-negative pneumonia following lung contusion. Shock. 2014;41:301–310. doi: 10.1097/SHK.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scicluna B.P., Baillie J.K. The search for efficacious new therapies in sepsis needs to embrace heterogeneity. Am J Respir Crit Care Med. 2019;199:936–938. doi: 10.1164/rccm.201811-2148ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seymour C.W., Kennedy J.N., Wang S., Chang C.H., Elliott C.F., Xu Z., et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321:2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shields A.M., Panayi G.S., Corrigall V.M. Resolution-associated molecular patterns (RAMP): RAMParts defending immunological homeostasis? Clin Exp Immunol. 2011;165:292–300. doi: 10.1111/j.1365-2249.2011.04433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neyra J.A., Li X., Canepa-Escaro F., Adams-Huet B., Toto R.D., Yee J., et al. Cumulative fluid balance and mortality in septic patients with or without acute kidney injury and chronic kidney disease. Crit Care Med. 2016;44:1891–1900. doi: 10.1097/CCM.0000000000001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphrey H., Hall J., Sznajder I., Silverstein M., Wood L. Improved survival in ARDS patients associated with a reduction in pulmonary capillary wedge pressure. Chest. 1990;97:1176–1180. doi: 10.1378/chest.97.5.1176. [DOI] [PubMed] [Google Scholar]
- 42.Chao W.C., Tseng C.H., Chien Y.C., Sheu C.C., Tsai M.J., Fang W.F., et al. Association of day 4 cumulative fluid balance with mortality in critically ill patients with influenza: a multicenter retrospective cohort study in Taiwan. PloS One. 2018;13 doi: 10.1371/journal.pone.0190952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbar S.D., Clere-Jehl R., Bourredjem A., Hernu R., Montini F., Bruyere R., et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379:1431–1442. doi: 10.1056/NEJMoa1803213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.