Abstract
Objectives
Superficial surgical site infection (SSSI) may increase the risk of serious complications such as periprosthetic joint infection (PJI). This study aims to identify patient-related risk factors associated with SSSI and investigate their correlation with the progression of PJI.
Design
In this retrospective study, 1191 elective hip and knee prostheses were included. Patients were interviewed 3–5 months after surgery to answer questions about the postoperative period. Patient records were reviewed to determine whether there had been any documentation of wound-healing difficulties or whether antibiotics were prescribed to treat an infection related to arthroplasty surgery.
Setting
Uppsala University Hospital, patients treated between November 2008 and December 2012.
Participants
The study population comprised 433 knees and 758 hips.
Outcome measures
We studied patient-related risk factors (joint, age, sex, the American Society of Anesthesiologists (ASA) classification, body mass index (BMI), smoking, diabetes and rheumatic disease) to determine whether they were associated with (1) SSSI and (2) the progress from SSSI to PJI.
Results
84 (7%) patients of the total cohort developed SSSI. This infection progressed to a PJI in 24 (29%) of the patients. Factors with increased adjusted risk ratios (aRRs) for SSSIs were knee surgery (1.7; 95% CI: 1.1 to 2.7), age≥65 years (1.7; 95% CI: 1.1 to 2.8), BMI≥30 (1.9; 95% CI: 1.0 to 3.4) and ASA classification≥3 (1.7; 95% CI: 1.0 to 2.9). ASA classification≥3 was the only factor showing a significant progression from SSSI to PJI (aRR=3.3; 95% CI: 1.0 to 10.3).
Conclusions
The risk of progressing from an SSSI to a PJI is high. Older patients, patients with obesity, and those with a high ASA classification considered for elective total knee arthroplasty seem to have an increased risk of developing SSSI. Patients with a high ASA classification seem to have an increased risk of progressing from SSSI to PJI.
Keywords: Musculoskeletal disorders, Adult orthopaedics, Hip, Knee
Strengths and limitations of this study.
Strengths of this study include the large cohort size (n=1191) and careful follow-up of each patient.
Exclusive inclusion of patients with primary elective arthroplasty surgery on the hip or knee.
Limitations of this study include the retrospective study design and the small number of infections, leading to a potential risk of a type 2 error.
Introduction
Infection after total joint arthroplasty (TJA) can be defined as either superficial involving skin or subcutaneous tissue only (a superficial surgical site infection (SSSI)) or deep (periprosthetic joint infection (PJI)) with deep soft tissue involvement (eg, fascial and muscle layers) and the prosthesis. The incidence of SSSI after TJA can vary from 1% to 10%1–3 and may increase the risk of subsequent PJI by 35-fold.3 The frequency of PJI ranges between 1% and 5%.4–7 Patient-related risk factors, such as obesity, rheumatoid arthritis (RA), smoking, male sex, age, alcohol abuse, American Society of Anesthesiologists (ASA) classification >2 and diabetes mellitus (DM),8–16 have been described as risk factors for PJI but not confirmed as risk factors for SSSI. In clinical practice, priority should be to identify high-risk patients for SSSI and PJI, aiming for optimal patient outcomes and the opportunity to manage modifiable risk factors. It is essential to seize any possibility to optimise all prerequisites for the best achievable surgical outcome. Thus, we sought to (1) determine which patient-related factors are linked to SSSI and (2) investigate the progression from SSSI to PJI.
Methods
Study design
This retrospective study of primary elective prostheses in hip or knee joints included patients in a national project designed to reduce the incidence of hospital-related infections.17 The study patients were treated at Uppsala University Hospital from November 2008 to December 2012 and interviewed 3–5 months after surgery to answer questions about the postoperative period. Patient medical records were reviewed to determine whether there had been any documentation of difficulties with wound healing or whether antibiotics were prescribed to treat an infection related to hip or knee arthroplasty. An orthopaedic consultant reviewed all information from patient records (recorded from general practitioners or orthopaedic consultants), including possible wound healing complications or antibiotic prescriptions due to suspected postoperative infection. This information and the results of the patient interview were used to determine the occurrence of SSSI. The diagnosis of PJI was made by a consultant orthopaedic surgeon in consultation with a consultant in infectious diseases. In a retrospective review of patient records selected patients met the criteria for PJI,18 but those criteria were not used at the time of diagnosis. Patient records were reviewed for patient-related risk factors, and a local arthroplasty register was used to obtain information about revision surgery that had been necessary due to persistent PJI. This study was limited to patient-related risk factors associated with the development of SSSI and focused on factors that may be avoidable or preoperatively optimised.
Study population
The study population comprised 664 men, 527 women and the study material included 1191 joints (433 knees, 758 hips). Hip prostheses were cemented, uncemented or hybrids. Only cemented components were used in all knees. In all cemented prostheses, antibiotic-loaded cement with gentamycin was applied. All patients received systemic preoperative and perioperative antibiotic prophylaxis in accordance with national guidelines (cloxacillin, and in the case of penicillin allergy, clindamycin).
Confounders
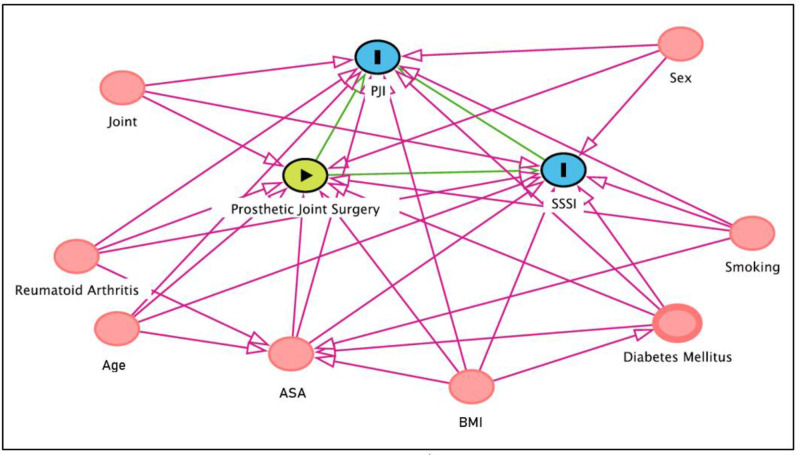
Patient-related factors (eg, joint, sex, age, body mass index (BMI), RA, ASA classification, smoking, DM) were considered clinically relevant for the association between arthroplasty and SSSI or PJI. These specific patient-related variables have previously been linked to exposure and outcome and are not considered in the causal pathway between potential risk factors and outcome (figure 1).
Figure 1.
Directed acyclic graph for the selection of confounders. The circle with an arrow indicates the exposure, the circles with an (I) illustrate outcomes and the circles without text indicate confounders used in the statistical model. ASA, American Society of Anesthesiologists; BMI, body mass index, PJI, periprosthetic joint infection; SSSI, superficial surgical site infection.
Relevant confounders were analysed as categorical variables: ASA classification (<3 or ≥3), age (<65 years or ≥65 years) and DM (patients with DM included both types 1 and 2, drug related or diet related). For BMI, the WHO classification was used and divided into the following groups: BMI<25 (under and normal weight), 25≤BMI<30 (overweight) and BMI≥30 (obesity classes 1–3).
Statistics
Descriptive statistics were used to summarise and report demographic characteristics.
In an initial analysis, logistic regression was performed, entering covariates as single variables. Crude risk ratios (RRs) for SSSI and PJI were calculated for each variable with 95% CIs. The covariates were entered into the regression model in the next step, with RRs mutually adjusted for all covariates. An adjusted RR (aRR) for each covariate was calculated for the occurrence of SSSI or PJI and any progression of SSSI to PJI.
All statistical analyses were performed using SPSS (V.26.0) and p values ≤0.05 were considered significant.
Patient and public involvement
Patients or the public were not involved in developing the research questions, outcome measures, the design, conduct, reporting or dissemination plans of our research. The results of this study will not be distributed separately to study participants.
Results
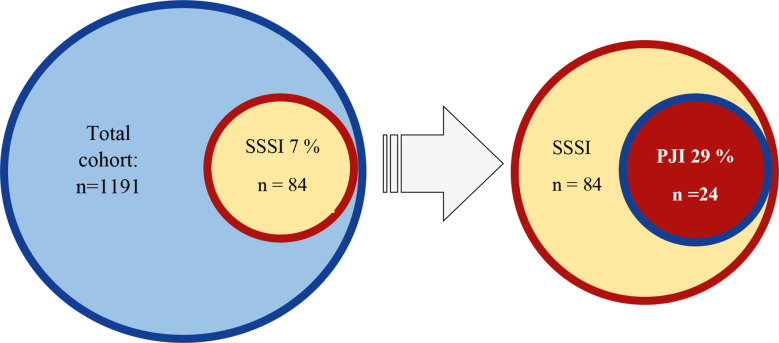
The number of surgeries for prosthetic hip or knee joints was 1191. Overall, 84 joints (7%) developed an SSSI and 24 (2%) a PJI. Of the 84 joints with SSSI, 24 (29%) progressed to a PJI (figure 2).
Figure 2.
Proportion of superficial surgical site infection (SSSI) and periprosthetic joint infection (PJI) in the total cohort.
In the hip cohort (758), 40 joints (5%) developed an SSSI and 11 (2%) a PJI. In the knee cohort (433), 44 joints (10%) developed an SSSI and 13 (3%) a PJI (table 1).
Table 1.
Number of postoperative infections
| Variable | SSSI | PJI |
| Total cohort (1191) | 84 (7.1%) | 24 (2.0%) |
| Hip (758) | 40 (5.3%) | 11 (1.5%) |
| Knee (433) | 44 (10.4%) | 13 (3.1%) |
PJI, periprosthetic joint infection; SSSI, superficial surgical site infection.
Demographic characteristics of the cohorts are outlined in table 2.
Table 2.
Cohort demographic characteristics
| Variable | Total cohort | Hip | Knee | Range |
| n=1191 | n=758 (64%) | n=433 (36%) | ||
| Mean age (year) | 63 | 61 | 65 | 18–96 |
| Age | ||||
| <65 | 673 (56%) | 447 (59%) | 226 (52%) | |
| ≥65 | 518 (44%) | 331 (41%) | 207 (48%) | |
| ASA class‡ | ||||
| ≤2 | 964 (81%) | 631 (84%) | 333 (78%) | |
| ≥3 | 210 (18%) | 117 (16%) | 93 (22%) | |
| Mean BMI | 28 | 27 | 29 | 14–51 |
| BMI | ||||
| BMI<25* | 356 (30%) | 259 (34%) | 97 (22%) | |
| 25≤BMI<30 | 474 (40%) | 307 (41%) | 167 (39%) | |
| BMI≥30 | 361 (30%) | 192 (25%) | 169 (39%) | |
| Sex | ||||
| Women | 664 (56%) | 387 (51%) | 277 (64%) | |
| Men | 527 (44%) | 371 (49%) | 156 (36%) | |
| Smoking† | ||||
| No | 1064 (90%) | 673 (89%) | 391 (90%) | |
| Yes | 122 (10%) | 81 (11%) | 41 (10%) | |
| Diabetes | ||||
| No | 1067 (90%) | 686 (91%) | 381 (88%) | |
| Yes | 124 (10%) | 72 (9%) | 52 (12%) | |
| Rheumatological disease | ||||
| No | 1052 (88%) | 690 (91%) | 362 (84%) | |
| Yes | 139 (12%) | 68 (9%) | 71 (16%) |
*Underweight 17 cases (BMI<18.5).
†Missing data in 5 cases.
‡Missing data in 17 cases.
ASA, American Society of Anesthesiologists; BMI, body mass index.
Risk factors for SSSI
Risk factors with a significant crude RR for developing SSSI were knee surgery (RR=2.0; 95% CI: 1.3 to 3.2), age≥65 years (RR=1.8; 95% CI: 1.2 to 2.8), ASA classification≥3 (RR=2.4; 95% CI: 1.5 to 3.8) and rheumatic disease (RR=1.9; 95% CI: 1.1 to 3.3). Adjusting for all covariates, factors with significant aRRs for SSSI were knee surgery (aRR=1.7; 95% CI 1.1 to 2.7), age≥65 years (aRR=1.7; 95% CI: 1.1 to 2.8), ASA classification≥3 (aRR=1.7; 95% CI: 1.0 to 2.9) and BMI≥30 (aRR=1.9; 95% CI: 1.0 to 3.4) (table 3).
Table 3.
Risk ratio (RR) for SSSI
| No SSSI | 1107 (9%) | ||||
| SSSI | 84 (7%) | ||||
| Variable | Crude RR (95% CI) | P value | Adjusted RR (95% CI) | P value | |
| Joint | |||||
| Hip | Ref | Ref | |||
| Knee | 2.0 (1.3 to 3.2) | 0.002 | 1.7 (1.1 to 2.7) | 0.022 | |
| Age | |||||
| <65 | Ref | Ref | |||
| ≥65 | 1.8 (1.2 to 2.8) | 0.010 | 1.7 (1.1 to 2.8) | 0.024 | |
| ASA class* | |||||
| ≤2 | Ref | Ref | |||
| ≥3 | 2.4 (1.5 to 3.8) | 0.001 | 1.7 (1.0 to 2.9) | 0.038 | |
| BMI | |||||
| BMI<25 | Ref | Ref | |||
| 25≤BMI<30 | 1.4 (0.8 to 2.2) | 0.211 | 1.1 (0.6 to 2.0) | 0.780 | |
| BMI≥30 | 2.3 (0.9 to 5.5) | 0.075 | 1.9 (1.0 to 3.4) | 0.045 | |
| Sex | |||||
| Women | Ref | Ref | |||
| Men | 1.1 (0.7 to 1.7) | 0.677 | 1.1 (0.8 to 2.1) | 0.298 | |
| Smoking† | |||||
| No | Ref | Ref | |||
| Yes | 1.1 (0.5 to 2.3) | 0.811 | 1.1 (0.5 to 2.3) | 0.841 | |
| Diabetes | |||||
| No | Ref | Ref | |||
| Yes | 1.6 (0.9 to 3.1) | 0.118 | 1.1 (0.6 to 2.2) | 0.753 | |
| Rheumatological disease | |||||
| No | Ref | Ref | |||
| Yes | 1.9 (1.1 to 3.3) | 0.031 | 1.7 (0.9 to 3.1) | 0.089 |
Numbers in bold mark statistical significance.
*Missing data in 17 cases.
†Missing data in 5 cases.
ASA, American Society of Anesthesiologists; BMI, body mass index.
Risk factors for PJI
The only risk factor with a significant crude RR for the development of PJI was ASA classification≥3 (RR=4.8; 95% CI: 2.1 to 10.9). Factors with significant aRRs for PJI were ASA classification≥3 (aRR=3.8; 95% CI: 1.6 to 9.1) and male sex (aRR=2.8; 95% CI: 1.2 to 6.9) (online supplemental table 1).
bmjopen-2022-060754supp001.pdf (42.3KB, pdf)
Risk factors for PJI in patients with SSSI
The only risk factor with a significant crude RR for the development of PJI in the SSSI group was ASA classification≥3 (RR=3.0; 95% CI: 1.1 to 8.1). The aRR shown for ASA classification was 3.3 (95% CI: 1.0 to 10.3) (table 4).
Table 4.
Risk ratio (RR) for PJI in patients with SSSI
| SSSI—no progression to PJI | 60 (71%) | ||||
| SSSI—progression to PJI | 24 (29%) | ||||
| Variable | Crude RR (95% CI) | P value | Adjusted RR (95% CI) | P value | |
| Joint | |||||
| Hip | Ref | Ref | |||
| Knee | 1.1 (0.4 to 2.9) | 0.836 | 1.3 (0.4 to 3.6) | 0.663 | |
| Age | |||||
| <65 | Ref | Ref | |||
| ≥65 | 1.5 (0.6 to 3.9) | 0.404 | 2.0 (0.7 to 6.2) | 0.212 | |
| ASA class | |||||
| ≤2 | Ref | Ref | |||
| ≥3 | 3.0 (1.1 to 8.1) | 0.030 | 3.3 (1.0 to 10.3) | 0.044 | |
| BMI | |||||
| BMI<25 | Ref | Ref | |||
| 25≤BMI<30 | 1.7 (0.6 to 5.0) | 0.309 | 2.3 (0.5 to 9.6) | 0.264 | |
| BMI≥30 | 1.2 (0.5 to 3.1) | 0.728 | 1.8 (0.5 to 7.1) | 0.411 | |
| Sex | |||||
| Women | Ref | Ref | |||
| Men | 2.5 (0.9 to 6.6) | 0.065 | 2.8 (0.9 to 8.3) | 0.064 | |
| Smoking | |||||
| No | Ref | Ref | |||
| Yes | 4.9 (0.5 to 51.1) | 0.188 | 5.3 (0.5 to 54.4) | 0.160 | |
| Diabetes | |||||
| No | Ref | Ref | |||
| Yes | 0.6 (0.2 to 2.0) | 0.394 | 1.3 (0.3 to 5.7) | 0.774 | |
| Rheumatological disease | |||||
| No | Ref | Ref | |||
| Yes | 1.2 (0.4 to 3.8) | 0.792 | 1.1 (0.3 to 4.7) | 0.883 | |
Numbers in bold mark statistical significance
ASA, American Society of Anesthesiologists; BMI, body mass index.
Discussion
Identifying, mitigating and optimising risk factors for SSSI and PJI are a desirable approach to prevent this devastating complication. The results of our study are relevant and provide new insight into the relationship between patient-related risk factors for SSSI and their association with the risk of PJI development. The risk and consequences of PJI after TJA have already been described.2 5 12 It has been reported that the occurrence of SSSI increases the risk of PJI by up to 35 times.3 However, factors affecting the progression from SSSI to PJI have not been investigated. Identifying and optimising risk factors for SSSI may decrease the risk of PJI.
Patient-related risk factors for SSSI
This study shows that knee surgery, age >65 years, a high ASA classification (≥3) and obesity (BMI>30) are independent risk factors for developing SSSI after elective primary joint arthroplasty.
Knee surgery seems to be a risk factor for developing SSSI after elective primary arthroplasty. Studies have shown that patients with knee prostheses have a higher risk of PJI and are in greater need of revision surgery than patients with hip prostheses.6 9 12 19 Because there is less soft tissue around the knee than around the hip, meaning a shorter distance between skin and joint, the risk of superficial infection is also increased. Blood circulation around the knee area is more exposed to impact than the hip area and perfusion is more easily disturbed. Increased tourniquet time has been identified as an individual risk factor for deep infection, impaired wound healing and prolonged wound discharge after total knee arthroplasty (TKA).1 However, due to a lack of data, this analysis could not be included in this study.
Our finding that age is a significant risk factor for SSSI is congruent with the results from a large (n=1000) retrospective study.1 Older patients (≥65 years) may have pre-existing medical conditions and fragile skin that could impair wound healing, making them more susceptible to SSSI.
The correlation between a high ASA Score and infection after surgery can be explained because the ASA classification encapsulates several other known risk factors (eg, smoking, DM, obesity). These risk factors have been independently associated with a higher risk of surgical site infection due to tissue hypoperfusion and subsequent impaired immunological function.13
Our obese patients have a 1.9 times higher risk of SSSI after primary elective arthroplasty. Shohat et al reported in a study including 19 000 patients that the risk for infection increases with higher BMI levels, although no BMI threshold was observed.20 Our results align with another large register-based study that reported an increased risk of reoperation in patients with higher BMI classification (obesity classes 1–3).21 The association between BMI and postoperative wound complications may be explained by linked comorbidities (eg, DM type 2),22 prolonged or more complicated arthroplasty surgery23 and protracted postoperative wound drainage.24 It has also been suggested that, although overweight and obese patients may not be calorie deficient, they are often micronutrient and protein deficient.25–28 Thus, malnutrition can be linked to increased BMI. Patients with preoperative malnutrition have higher rates of comorbidities (congestive heart failure, previous cardiac surgery, hypertension, dyspnoea, chronic obstructive pulmonary disease, renal disease requiring dialysis, stroke, diabetes, chronic corticosteroid use, bleeding disorders).29 Higher rates of surgical site infection after TJA are shown in patients with hypoalbuminaemia.30
It has been described that RA is a risk factor for developing PJI after TJA. A systematic review presented a significant OR for PJI in patients with RA with a THA of 1.75 (95% CI: 1.49 to 2.06)12 and an OR of 1.34 (95% CI: 1.18 to 1.52) in patients with a TKA. We found that RA had a significant crude RR for developing SSSI after primary TJA. The risk was 1.7 times higher for patients with RA than those without RA when adjusting for all covariates, although this risk difference was not statistically significant (table 3). The significance of the association between RA and SSSI may have gone undetected in this analysis because of the small number of infections and type 2 statistical error.
Patient-related risk factors for the development of deep surgical site infection
We found that superficial wound complications were associated with developing PJI in 29% of our patients, with high ASA classification (3.3 times higher than patients with ASA<3) a determining factor in the progression from SSSI to PJI. ASA classification is a crude estimate of a patient’s medical condition and a high score has been associated with the risk of PJI in numerous reports.11 12 For instance, Blanco et al reported a 15-fold OR (95% CI: 6.54 to 35.80) for PJI in patients with ASA classification≥3 31 and Panula et al presented an HR of 3.2 (95% CI: 2.0 to 5.1) for the same ASA classification.32
A recent meta-analysis showed that male sex was a risk factor for PJI development, especially after TKA.19 In our analysis, male sex was close to a significant risk factor for progression from SSSI into PJI, both as a single variable and after adjustment for all covariates (2.8 times higher risk for men than women). The link between male sex and PJI may be attributed to certain contributing behavioural factors, including smoking, diet, hygiene and alcohol consumption, but the underlying reasons for this are unclear. Sex-related differences in immune response due to bacteria (eg, Staphylococcus aureus and Pseudomonas aeruginosa) have been reported. In addition, septicaemia and bacteraemia occur more frequently in males than females,33 but whether they will affect the development of SSSI or PJI has yet to be determined. The absence of statistical significance for male sex as a risk factor of developing PJI after SSSI can depend on the total number of infected patients in our study.
Prevention of postoperative infection
The rate of SSSI (7%) and PJI (2%) in this study is consistent with international studies showing levels of SSSI ranging from 1% to 10%1 34 and PJI ranging from 0.2% to 2.23%.5 6 8 The present work is focused on patient-related factors with a possible effect on the occurrence of SSSI or PJI after elective primary TJA. Several other factors related to the surgery (eg, operation time, intraoperative blood loss, number of door openings, discipline in the operating room, antibiotic prophylaxis used, surgeon’s experience) may affect the risk of postoperative infection. However, those factors are not included in our analysis. With the challenging complexity of PJI and its heavy burden on patients35 and healthcare systems,36 prevention through effective strategies is the first and best approach and should be prioritised. Identifying high-risk patients planning to undergo arthroplasty surgery and providing interventions when possible by modifying these risk factors might form the basis of PJI prevention strategies in the future.
Strengths
Two major strengths of this study are the large sample size (n=1191) and the meticulous follow-up of each patient. This thorough postoperative follow-up confirms that the number of recorded incidents of SSSI is accurate, and the follow-up time of 5 (mean 7.3; range 5.1–9.2) years is sufficient to reveal potential cases of PJI. Similar studies have presented larger cohorts but only on registers37 38 or shorter follow-ups.1 2 37
Additionally, our study includes patients with primary elective joint surgery to minimise the influence of other risk factors connected to the initial trauma (hip fractures) or extended impact on the tissue (revision surgery). This inclusion criterion is an additional strength of the study given that the rate of PJI is known to be higher after trauma and revision surgery.39 According to preoperative screening routines in our hospital, patients with a history of excessive alcohol use, intravenous drug use, poor oral hygiene or other medical conditions or medications that compromise immunity are excluded from surgery or rehabilitated before surgery.
Limitations
A potential limitation is the retrospective nature of our study design. Therefore, there may be inaccuracies or misinterpretations of information retrieved from medical records. All our patients, however, were interviewed in person to answer questions about the postoperative period. Thus, the information from medical reports was verified.
Another limitation is that SSSI is not culture-verified but determined by a consultant orthopaedic surgeon that reflects the clinical reality. Cultures of a superficial infection can be misleading, which can be classified as contamination, even if a possibility of a clinical significance of skin flora found in cultures has recently been reported.40
As in infection-related research in general, where a small number of infections are a major challenge, this study may have failed to detect an association between a potential risk factor and postoperative infection due to a type 2 error.
Conclusion
In conclusion, this study demonstrates that older obese patients with a high ASA classification considered for elective total knee arthroplasty may have an increased risk of developing SSSI. Patients developing SSSI after primary elective hip or knee arthroplasty have a high risk of progressing into PJI and a high ASA classification significantly affects the progression from SSSI to PJI.
Supplementary Material
Acknowledgments
We thank Jakob Viklander for his valuable assistance during the data compilation.
Footnotes
Contributors: Both authors (HKE, SL) made substantial contributions to conception and design of the study and to acquisition, analysis and interpretation of data. Both authors (HKE, SL) have been involved in drafting the manuscript and given final approval of the version to be published. Both authors (HKE, SL) have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. HKE accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Consent for publication was considered in the application to the Human Research Ethics Committee. Because this was a retrospective study, no consent (written or verbal) was needed for this work. The study design was reviewed and approved by the Human Research Ethics Committee (Dnr: 2019-01425).
References
- 1.Carroll K, Dowsey M, Choong P, et al. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect 2014;20:130–5. 10.1111/1469-0691.12209 [DOI] [PubMed] [Google Scholar]
- 2.Peersman G, Laskin R, Davis J, et al. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res 2001;392:15–23. [PubMed] [Google Scholar]
- 3.Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res 2002;20:506–15. 10.1016/S0736-0266(01)00153-X [DOI] [PubMed] [Google Scholar]
- 4.Lima ALL, Oliveira PR, Carvalho VC, et al. Periprosthetic joint infections. Interdiscip Perspect Infect Dis 2013;2013:1–7. 10.1155/2013/542796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty 2010;25:1216–22. 10.1016/j.arth.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 6.Ridgeway S, Wilson J, Charlet A, et al. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br 2005;87:844–50. 10.1302/0301-620X.87B6.15121 [DOI] [PubMed] [Google Scholar]
- 7.Cooper K, Lamagni T, Harrington P, et al. Surveillance of surgical site infections in NHS hospitals in England, April 2018 to March 2019. Available: www.gov.uk/phe
- 8.Phillips JE, Crane TP, Noy M, et al. The incidence of deep prosthetic infections in a specialist orthopaedic Hospital: a 15-year prospective survey. J Bone Joint Surg Br 2006;88:943–8. 10.1302/0301-620X.88B7.17150 [DOI] [PubMed] [Google Scholar]
- 9.Pulido L, Ghanem E, Joshi A, et al. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 2008;466:1710–5. 10.1007/s11999-008-0209-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty 2010;25:87–92. 10.1016/j.arth.2008.10.013 [DOI] [PubMed] [Google Scholar]
- 11.Dale H, Fenstad AM, Hallan G, et al. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop 2012;83:449–58. 10.3109/17453674.2012.733918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong L, Cao J, Zhang Y, et al. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: a meta-analysis. Int Wound J 2017;14:529–36. 10.1111/iwj.12640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunutsor SK, Whitehouse MR, Blom AW, et al. Patient-Related risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. PLoS One 2016;11:e0150866. 10.1371/journal.pone.0150866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maoz G, Phillips M, Bosco J, et al. The Otto Aufranc Award: modifiable versus nonmodifiable risk factors for infection after hip arthroplasty. Clin Orthop Relat Res 2015;473:453–9. 10.1007/s11999-014-3780-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everhart JS, Altneu E, Calhoun JH. Medical comorbidities are independent preoperative risk factors for surgical infection after total joint arthroplasty. Clin Orthop Relat Res 2013;471:3112–9. 10.1007/s11999-013-2923-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian arthroplasty register. Arthritis Care Res 2010;62:473–9. 10.1002/acr.20036 [DOI] [PubMed] [Google Scholar]
- 17.Swedish association of local authorities and regions, 2020. Available: www.skr.se [Accessed cited 15 May 2020].
- 18.Parvizi J, Tan TL, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 2018;33:1309–14. 10.1016/j.arth.2018.02.078 [DOI] [PubMed] [Google Scholar]
- 19.Resende VAC, Neto AC, Nunes C, et al. Higher age, female gender, osteoarthritis and blood transfusion protect against periprosthetic joint infection in total hip or knee arthroplasties: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 2021;29:8-43. 10.1007/s00167-018-5231-9 [DOI] [PubMed] [Google Scholar]
- 20.Shohat N, Fleischman A, Tarabichi M, et al. Weighing in on body mass index and infection after total joint arthroplasty: is there evidence for a body mass index threshold? Clin Orthop Relat Res 2018;476:1964–9. 10.1007/s11999.0000000000000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayed-Noor AS, Mukka S, Mohaddes M, et al. Body mass index is associated with risk of reoperation and revision after primary total hip arthroplasty: a study of the Swedish hip arthroplasty register including 83,146 patients. Acta Orthop 2019;90:220–5. 10.1080/17453674.2019.1594015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowsey MM, Choong PFM. Obesity is a major risk factor for prosthetic infection after primary hip arthroplasty. Clin Orthop Relat Res 2008;466:153–8. 10.1007/s11999-007-0016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel VP, Walsh M, Sehgal B, et al. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am 2007;89:33–8. 10.2106/00004623-200701000-00005 [DOI] [PubMed] [Google Scholar]
- 25.Cross MB, Yi PH, Thomas CF, et al. Evaluation of malnutrition in orthopaedic surgery. J Am Acad Orthop Surg 2014;22:193–9. 10.5435/JAAOS-22-03-193 [DOI] [PubMed] [Google Scholar]
- 26.Yayac M, Aggarwal R, Parvizi J. How viable is pre-surgery weight reduction for the reduction of periprosthetic joint infection risk after total joint arthroplasty? Expert Rev Med Devices 2020;17:149–51. 10.1080/17434440.2020.1720509 [DOI] [PubMed] [Google Scholar]
- 27.Nelson CL, Elkassabany NM, Kamath AF, et al. Are associated with complications after TKA. Clin Orthop Relat Res 2015;473:3163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia GH, Fu MC, Dines DM, et al. Malnutrition: a marker for increased complications, mortality, and length of stay after total shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:193–200. 10.1016/j.jse.2015.07.034 [DOI] [PubMed] [Google Scholar]
- 29.Fu MC, D'Ambrosia C, McLawhorn AS, et al. Malnutrition increases with obesity and is a stronger independent risk factor for postoperative complications: a Propensity-Adjusted analysis of total hip arthroplasty patients. J Arthroplasty 2016;31:2415–21. 10.1016/j.arth.2016.04.032 [DOI] [PubMed] [Google Scholar]
- 30.Bohl DD, Shen MR, Kayupov E, et al. Hypoalbuminemia independently predicts surgical site infection, pneumonia, length of stay, and readmission after total joint arthroplasty. J Arthroplasty 2016;31:15–21. 10.1016/j.arth.2015.08.028 [DOI] [PubMed] [Google Scholar]
- 31.Blanco JF, Díaz A, Melchor FR, et al. Risk factors for periprosthetic joint infection after total knee arthroplasty. Arch Orthop Trauma Surg 2020;140:239–45. 10.1007/s00402-019-03304-6 [DOI] [PubMed] [Google Scholar]
- 32.Panula VJ, Alakylä KJ, Venäläinen MS, et al. Risk factors for prosthetic joint infections following total hip arthroplasty based on 33,337 hips in the Finnish arthroplasty register from 2014 to 2018. Acta Orthop 2021;92:665–72. 10.1080/17453674.2021.1944529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClelland EE, Smith JM. Gender specific differences in the immune response to infection. Arch Immunol Ther Exp 2011;59:203–13. 10.1007/s00005-011-0124-3 [DOI] [PubMed] [Google Scholar]
- 34.Guirro P, Hinarejos P, Pelfort X, et al. Long term follow-up of successfully treated superficial wound infections following TKA. J Arthroplasty 2015;30:101–3. 10.1016/j.arth.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 35.Andersson AE, Bergh I, Karlsson J, et al. Patients' experiences of acquiring a deep surgical site infection: an interview study. Am J Infect Control 2010;38:711–7. 10.1016/j.ajic.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 36.Kurtz SM, Lau EC, Ong KL, et al. Which clinical and patient factors influence the National economic burden of hospital readmissions after total joint arthroplasty? Clin Orthop Relat Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almustafa MA, Ewen AM, Deakin AH, et al. Risk factors for surgical site infection following lower limb arthroplasty: a retrospective cohort analysis of 3932 lower limb arthroplasty procedures in a high volume arthroplasty unit. J Arthroplasty 2018;33:1861–7. 10.1016/j.arth.2018.01.037 [DOI] [PubMed] [Google Scholar]
- 38.Jämsen E, Huhtala H, Puolakka T, et al. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am 2009;91:38–47. 10.2106/JBJS.G.01686 [DOI] [PubMed] [Google Scholar]
- 39.Kärrholm J, Rogmark C, Nauclér E, et al. Swedish hip arthroplasty register. annual report, 2018. Available: www.shpr.se
- 40.Widerström M, Stegger M, Johansson A, et al. Heterogeneity of Staphylococcus epidermidis in prosthetic joint infections: time to reevaluate microbiological criteria? Eur J Clin Microbiol Infect Dis 2022;41:87–97. 10.1007/s10096-021-04352-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-060754supp001.pdf (42.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.