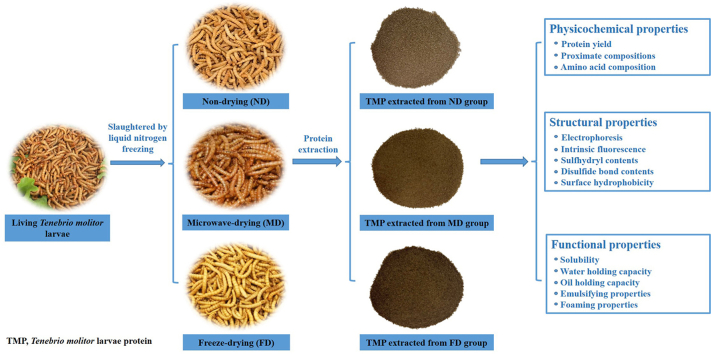
Abstract
Microwave drying (MD) or freeze drying (FD) was commonly used as a drying treatment prior to the extraction of edible insect proteins. However, some quality defects (e.g., lipid oxidation or protein denaturation) were probably occurred via the drying step. To this end, the effect of drying or non-drying treatments (ND) after slaughtering by liquid nitrogen freezing on the physicochemical characteristics, structural and functional properties of Tenebrio molitor larvae protein (TMP) was investigated. The results indicate that TMP extracted from the ND group showed higher essential/total amino acid content, total/free sulfhydryl content, surface hydrophobicity, solubility, water/oil holding capacities, and emulsifying/foaming properties than those extracted from the MD or FD groups (P < 0.05). Moreover, the ND group had minimal impact on the structural changes of TMP which was associated with protein denaturation. Therefore, it can be concluded that a non-drying strategy prior to TMP extraction can improve functional properties and retard protein denaturation, while also conserving energy.
Keywords: Tenebrio molitor larvae, Insect protein, Pre-extraction treatment, Structural properties, Functional properties
Graphical abstract
Highlights
-
•
Tenebrio molitor larvae was firstly slaughtered by liquid nitrogen freezing.
-
•
Frozen larvae were subjected to either drying or non-drying treatment.
-
•
Tenebrio molitor larvae protein (TMP) was extracted from dried or non-dried group.
-
•
TMP extracted from the non-dried group had optimum functional properties.
-
•
TMP extracted from the non-dried group had least degree of protein denaturation.
1. Introduction
It is well known that with rapid population growth all over the world, meeting the demand for food will become a daunting challenge. In particular, the demand for animal protein has increased, mainly in the form of meat products, which are expected to increase by 200 million tons annually by 2050 (Kim et al., 2016). Thus, there is an urgent need to increase the supply of animal protein from new and sustainable sources. Edible insects have great potential in dealing with food crises due to their diversity, apparent abundance and low environmental impact when considering large-scale production (Sánchez-Muros et al., 2014; Yen, 2015; Payne et al., 2016). In the last decade, edible insects have gained more attention as sustainable protein sources in the food ingredient supply chain of the food industry. Numerous studies have suggested that edible insects should be applied to food products in less recognizable forms (such as whole insect flour or extracted protein powder) to increase the overall acceptability of wary consumers (Kim et al., 2016; Zielińska et al., 2018; Gravel and Doyen, 2020). Moreover, the presence of unsaturated fatty acids and chitin may induce poorer oxidative stability and lower digestibility of whole insect flour-added food products (van Huis, 2013; Kim et al., 2016). As a result, extracted or refined edible insect proteins have been recognized as potential ingredients for their excellent functional benefits (such as emulsifying capacities and gel-forming properties), as well as their high nutritional value and digestibility (Gravel and Doyen, 2020).
In general, the raw insects were currently slaughtered via blanching or freezing treatment (Leni et al., 2019). Blanching could effectively inactivate enzymes and destroy the microorganisms of the insects. However, the blanching treatment is rarely applied as a potential slaughtering method by entomophagy companies (Vandeweyer et al., 2017). Moreover, as indicated by Wessels et al. (2020), freezing is considered the most ethical and convenient strategy to kill insects for food uses, as they could die without regaining consciousness. Currently, the insects used for food purposes are commonly slaughtered via slowly freezing by most insect-rearing companies (Sun-Waterhouse et al., 2016). However, Leni et al. (2019) pointed out that slow freezing did not efficiently inhibit the enzymes presented in the insect biomass, and subsequently led to more black protein fractions after extraction. Gao et al. (2019) also indicated that large-size ice crystals could be easier formed by slowly freezing and then promoted the breakdown of the cell membrane, leading to the quality deterioration of extracted proteins. Recently, liquid nitrogen freezing has become a potential method for rapid food freezing. Compared with conventional slowly freezing, liquid nitrogen freezing exhibited an extremely fast freezing rate, mainly attributed to its low heat resistance, and showed decreased production cost and high food quality (Yang et al., 2022). Thus, the application of liquid nitrogen freezing with a higher freezing rate and freezing efficiency can be considered a crucial method for the improved quality of extracted edible insect proteins.
Subsequently, before the extraction process of obtaining edible insect proteins, drying is an essential and common step to reduce the weight of insects and the costs of transportation or storage (Lenaerts et al., 2018). Meanwhile, the drying treatment could reduce water activity to increase the microbial stability of the larvae. As indicated by Melgar-Lalanne et al. (2019), some traditional or modern drying methods (such as solar-drying, oven-drying, smoke-drying, freeze-drying, microwave-drying, fluidized bed drying, etc.) have been commonly used to dry whole insects before initiating a grinding procedure. However, lipid oxidation and color could be obviously impaired by the different drying methods. For instance, Megido et al. (2018) indicated that solar-drying and smoke-drying could significantly promote the degree of lipid oxidation and color degradation of dried edible insects. Freeze-drying could effectively maintain good raw material quality, but with significant energy costs. However, freeze-drying could also promote lipid oxidation and decreased solubility of extracted proteins (Melgar-Lalanne et al., 2019). Kröncke et al. (2018) also found that microwave-drying could substantially reduce the solubility of mealworm proteins, mainly due to the structural changes induced by microwaves. Moreover, Leni et al. (2019) indicated that the different processing treatments could have diverse effects on the quality profiles of extracted edible insect proteins, such as protein yield, basic compositions, and functional properties. Until now, maintaining the original edible insect protein structural and functional properties post-extraction while eliminating the impairments induced by pre-extraction treatments are challenging hurdles to overcome. Thus, we hypothesized that directly grinding the non-dried edible insects after slaughtering by liquid nitrogen and following with a protein extraction process might be a more promising alternative to dried edible insects for obtaining high-quality edible insect proteins. However, available information on the effect of drying or not on the characteristics and functionalities of edible insect proteins is scarce.
Therefore, in our present study, fresh mealworm larvae (Tenebrio molitor L.) were firstly slaughtered with liquid nitrogen and then divided into three groups as follows: group (1) was used as a non-dried group (ND), and group (2) and (3) were dried either by microwave-drying (MD) or by freeze-drying (FD), and subsequently underwent associated extraction procedures to obtain Tenebrio molitor protein (TMP). The TMP received from different pre-extraction treatments (ND, MD, and FD) were then systematically and comparatively investigated for physicochemical, structural, and functional properties.
2. Material and methods
2.1. Insect samples
Tenebrio molitor larvae were purchased from Chongyuexuan Trading Co., Ltd. (Qingdao, Shandong, China) and delivered to the laboratory by keep-alive transportation. Prior to pretreatment, feed residue impurities were separated from living Tenebrio molitor larvae by passing the residue through a 20-mesh sieve. All other reagents and chemicals used were of analytical grade.
2.2. Pre-extraction treatments
For each batch, all the fresh Tenebrio molitor larvae (5.0 kg) were immediately frozen by using liquid nitrogen with the mass (the larvae) to volume (the liquid nitrogen) ratio of 1:2 for at least 30 s. After that, the frozen larvae were divided into three groups as follows: a group was used as a “non-dried” group, and the other two groups were dried by microwave or freeze-drying.
MD and FD of Tenebrio molitor larvae were performed according to the methods of Kröncke et al. (2018) with slight modifications. For microwave drying treatment, the frozen Tenebrio molitor larvae were placed on a 45 × 35 × 3 cm bottom plate (H6100, Miele, Germany) and then dried at 850 W for 10 min. For freeze-drying treatment, the frozen Tenebrio molitor larvae were placed in a freeze-dryer (Pilot 3–6M, Biocool Experimental Equipment Co. Ltd., Beijing, China) for 52 h.
2.3. Protein extraction from non-dried samples
The frozen Tenebrio molitor larvae from the non-dried group were directly ground via a grinder (DFT-50, Dade Co., Ltd., Taizhou, Zhejiang, China) and dispersed in distilled water with the mass to volume ratio of 1:3 (w/v). After that, sodium bisulfite (0.5 g/kg, based on the weight of insects) was added to inhibit enzymatic browning and stirred well at 500 rpm using an IKA mechanical mixer for 1 min. The above suspension was subjected to further protein extraction procedures.
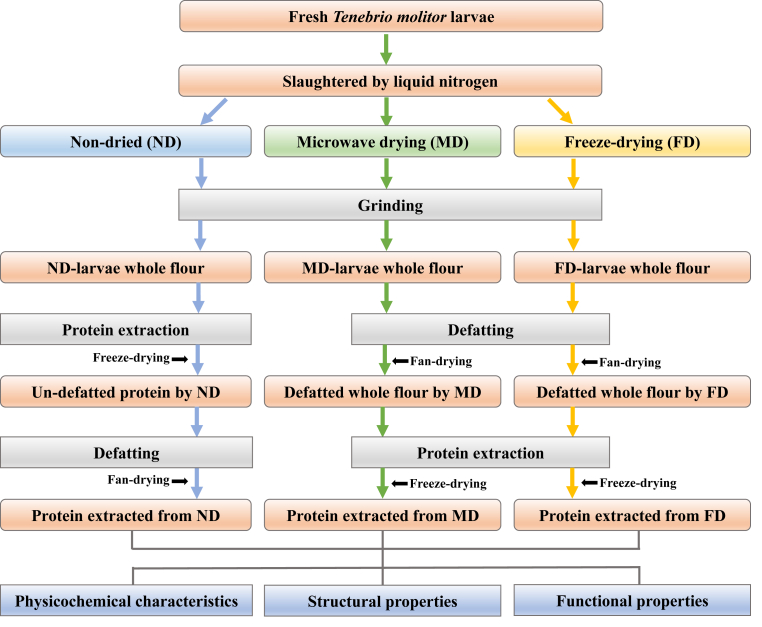
The detailed protein extraction flow chart is shown in Fig. 1 with steps as follows: 1) the pH of the suspension was adjusted to 10.0 with 1M NaOH, and stirred at 500 rpm using an IKA mechanical mixer for 1 h, and then centrifuged at 5000 g for 20 min at 4 °C to obtain a supernatant and precipitate, 2) the supernatant was collected, and the above procedure was repeated twice for the precipitate, 3) the combined supernatant pH (all supernatants were combined) was adjusted to an isoelectric point (pI, pH = 4.30–4.50) with 1.0 M HCl and incubated for 1h at room temperature (25 °C), and then centrifuged at 5000 g for 20 min at 4 °C, 4) the obtained precipitate was collected and re-dissolved in deionized water and the pH was adjusted to 7.0 using 1 mol/L NaOH, 5) The obtained protein solutions were dialyzed with molecular weight cut-off 1000 Da 24 h at 4 °C and freeze-dried using a freeze-dryer (Pilot 3–6M, Biocool Experimental Equipment Co. Ltd., Beijing, China), 6) the insect protein was dispersed in n-hexane with a mass to volume ratio of 1:15 (w/v) and stirred at 500 rpm by using an IKA mechanical mixer for 1 h, and then centrifuged at 3000 g for 20 min at 4 °C to remove the fat, 7) the defatted insect protein was collected, and the above procedure was repeated 4 times, 8) the obtained TMP was placed in a fume hood overnight to remove the residual n-hexane, 9) dry TMP was well ground by a coffee grinder (type 203, Krups, Medford, MA) and passed through a 50-mesh sieve. Moreover, the n-hexane collected (steps 6 or 7) after centrifugation was recovered by a rotary evaporator (N-1001, Tokyo Rikakikai Co., Ltd. Tokyo, Japan) for cyclic utilization.
Fig. 1.
The flow chart of protein extraction from Tenebrio molitor larvae with different pre-extraction treatments.
2.4. Protein extraction from dried samples
The dried Tenebrio molitor larvae via MD or FD were first ground using a grinder (DFT-50, Dade Co., Ltd., Taizhou, Zhejiang, China) and then defatted according to the method of Kim et al. (2020). Briefly, the ground Tenebrio molitor larvae flour was dispersed in n-hexane with the mass to volume ratio of 1:5 (w/v) and stirred at 500 rpm using an IKA mechanical mixer for 1 h to remove the fat, and then centrifuged at 5000 g for 5 min at 4 °C to obtain defatted Tenebrio molitor larvae flour. Moreover, to remove the fat as much as possible, the above procedure was repeated 4 times. After that, the collected defatted Tenebrio molitor larvae flour was placed in a fume hood overnight to remove the residual n-hexane. In addition, the n-hexane collected in the defatting procedure after centrifugations were recovered by a rotary evaporator (N-1001, Tokyo Rikakikai Co., Ltd. Tokyo, Japan) for cyclic utilization.
Before protein extraction of TMP, the defatted Tenebrio molitor larvae flour was first dispersed in distilled water with the mass to volume ratio of 1:15 (w/v). After that, sodium bisulfite (0.5 g/kg, based on the weight of insects) was added to inhibit enzymatic browning and stirred well at 500 rpm by using an IKA mechanical mixer for 1 min. The above suspension was subjected to further protein extraction procedures.
The detailed protein extraction flow chart is shown in Fig. 1 as follows: 1) the pH of the suspension was adjusted to 10.0 with 1M NaOH, and stirred at 500 rpm using an IKA mechanical mixer for 1 h, and then centrifuged at 5000 g for 20 min at 4 °C to obtain a supernatant and the precipitate, 2) the supernatant was collected, and the above procedure was repeated twice for the precipitate, 3) the combined supernatant pH (all supernatants were combined) was adjusted to an isoelectric point (pI, pH = 4.30–4.50) (Gould and Wolf, 2018) with 1.0 M HCl and incubated for 1h at room temperature (25 °C), and then centrifuged at 5000 g for 20 min at 4 °C, 4) the obtained precipitate was collected and re-dissolved in deionized water and the pH was adjusted to 7.0 using 1 mol/L NaOH, 5) The obtained protein solutions were dialyzed with molecular weight cut-off 1000 Da 24 h at 4 °C and freeze-dried using a freeze-dryer (Pilot 3–6M, Biocool Experimental Equipment Co. Ltd., Beijing, China), 6) dry TMP were well ground by a coffee grinder (type 203, Krups, Medford, MA) and passed through a 50-mesh sieve.
2.5. Protein yield
The protein concentrations in TMP were measured using the method described in the section on “proximate compositions”. The mass of TMP extracted from Tenebrio molitor larvae with different pretreatment methods was calculated by multiplying the measured protein concentration by the weight of TMP. The protein yield was calculated as follows:
where WP is the weight of the TMP, CP is the concentration of the TMP, WT is the weight of the Tenebrio molitor flours, and CTP is the protein concentration of the Tenebrio molitor flours. All weights were calculated on a dry base.
2.6. Physicochemical properties
2.6.1. Proximate compositions
The proximate compositions (such as moisture, protein, fat, and ash content) of coarse Tenebrio molitor flour or TMP were measured according to the method of Lenaerts et al. (2018).
2.6.2. Amino acid composition
Amino acid compositions of TMP were determined according to the method of Kim et al. (2020) and Yi et al. (2013) by using an amino acid analyzer (L-8800, Hitachi, Japan). Protein quality was assessed via the essential amino acid index (EAAI), which was calculated as follows:
The human essential amino acids were used as a reference protein (Rf.) for which to refer (FAO/WHO/UNU, 1985).
2.6.3. Particle size
The particle sizes of TMP solutions (1.0 mg/mL) were measured according to the method of Jiang et al. (2020) via a laser particle size analyzer (Malvern Instruments Ltd., Worcestershire, UK) with slight modification. Test parameters: scattering angle: 90°, balance time: 60 s, test temperature: 25 °C. Particle size was expressed as D (3,2), and D (4,3), where D (3,2) represents volume-surface mean diameter and D (4,3) represents the volume-mean diameter.
2.6.4. Color
The color of TMP was determined using a ZE-6000 color meter (Nippon Denshoku, Kogyo Co., Ltd. Tokyo, Japan), to produce a L*-value, a*-value and b*-value. Moreover, according to Mohapatra et al. (2010), the ΔE*-value and browning index (BI) were calculated as follows:
2.7. The structural properties of TMP
2.7.1. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE of each TMP sample was performed by using a 5.0% stacking gel and a 10.0% running gel using a Mini-Protean II cell electrophoresis system (Bio-Rad Laboratories, Richmond, CA, USA) according to the method of Liu et al. (2014) under reducing (electrophoresis samples prepared with β-mercaptoethanol) or non-reducing (electrophoresis samples prepared without β-mercaptoethanol) conditions with some modifications. Briefly, 1.0 mL of each TMP solution (2.0 mg/mL) was mixed with 1.0 mL of the sample buffer (containing 4% SDS, 20% glycerol, with or without 10% β-mercaptoethanol, and 0.125 M Tris-HCl, pH 6.8), and heated to 100 °C for 5 min, and then centrifuged at 1800 g to remove any particulates. Aliquots of 20 μL of the supernatants were loaded into each well on the gel. A molecular weight (MW) standard, composed of a cocktail of proteins (from 11 kDa to 245 kDa) (TaKaRa Biotechnology Co., Ltd. Dalian, China) was also run.
2.7.2. Determination of total and exposed sulfhydryl contents
The total and exposed sulfhydryl contents of TMP were determined using the same procedure as Liu et al. (2015). Briefly, 0.5 mL of each TMP solution (2.0 mg/mL) was mixed with 4.5 mL reaction buffer (0.09 M glycine, 0.086 M Tris, and 4 mM EDTA at pH 8.0) with (for total sulfhydryl contents) or without (for exposed sulfhydryl contents) 8 M urea. After adding 50 μL of Ellman's reagent, the resultant suspensions were incubated for 1 h at room temperature (25 °C) with occasional vibrating and then centrifuged for 15 min at l0,000 g. The absorbance of the supernatant was read at 412 nm with the reagent buffer as the blank. The total sulfhydryl and exposed sulfhydryl contents in micromoles per gram of protein were calculated using the extinction coefficient of NTB, which is 13,600 M−1 cm−1.
2.7.3. Determination of disulfide bond contents
Disulfide bond content of TMP was measured according to the method of Gong et al. (2016) with some modifications as follows: 1) TMP powder (60 mg) was added to 10 mL Tris-glycine buffer (0.086 M Tris, 0.09 M glycine, 4 mM EDTA, 8 M urea, pH 8.0) and then stirred at 500 rpm for 30 min via a magnetic stirrer. After that, the solution was centrifuged at 10,000 g for 10 min, and the supernatant was collected. 2) Measure the sulfhydryl content of the TMP sample without β-mercaptoethanol. Briefly, 0.16 mL of Ellman's reagent (4.0 mg/mL) was added to 4.0 mL of supernatant from step 1, and incubated in a dark room at room temperature (25 °C) for 30 min, and followed by centrifugated at 10,000 g for 10 min. The absorbance of the supernatant was measured at 412 nm with the reagent buffer as control. 3) Measure the sulfhydryl content of the TMP sample with β-mercaptoethanol. Briefly, 8 μL of β-mercaptoethanol was added to 4.0 mL of supernatant from step 1 and then incubated for 2 h at room temperature (25 °C). After that, 10 mL of trichloroacetic acid (12%, w/v) was added and incubated for 1 h at room temperature (25 °C) and then centrifugated at 10,000 g for 10 min. The precipitate was resuspended in 6.0 mL of Tris-glycine buffer and added with 0.24 mL of Ellman's reagent (4 mg/mL), and incubated in dark room at room temperature (25 °C) for 30 min, and then followed by centrifugated at 10,000 g for 10 min. The absorbance of the supernatant was measured at 412 nm with the reagent buffer as control. 4) The sulfhydryl contents of TMP with or without β-mercaptoethanol in micromoles per gram of protein were calculated using the extinction coefficient of NTB, which is 13,600 M−1 cm−1 5) The disulfide content of TMP was calculated as follows:
where, C1 is the sulfhydryl contents of TMP samples in the absence of β-mercaptoethanol; C2 is the sulfhydryl contents of TMP samples in the presence of β-mercaptoethanol.
2.7.4. Determination of surface hydrophobicity
The surface hydrophobicity of each TMP was determined according to the method of Chelh et al. (2006) with some modifications. Briefly, 200 μL of bromophenol blue (BPB, 1 mg/mL) was added to 1 mL of TMP solution (1.0 mg/mL), and then centrifuged at 6000 g for 15 min at 4 °C. After that, the supernatant was diluted by a factor of 10, and the absorbance was measured at 595 nm. A 10 mM pH 7.0 phosphate buffered solution (PBS) was used as a blank. The surface hydrophobicity of each sample was calculated as follows:
where A blank is the absorbance of the blank sample, A sample is the absorbance of the TMP sample.
2.7.5. Fourier transform infrared (FTIR) spectroscopy
FTIR spectra of TMP were recorded by a Nicolet IS 50 (Thermo Fisher Scientific Co., Ltd, German). The TMP sample was pressed into 1–2 mm slice and analyzed. The scanning wavenumber was between 400 and 4000 cm−1. Data analysis was done utilizing OMNIC and PF (Peakfit) software.
2.7.6. Fluorescence measurement
According to the same method as Feng et al. (2021), the intrinsic tryptophan fluorescence of each TMP sample solution (1.0 mg/mL) was measured using a fluorescence spectrophotometer (F-4500, Hitachi, Tokyo, Japan) under the excitation wavelength of 295 nm.
2.8. The functional properties of TMP
2.8.1. Solubility
The solubility of each TMP at different pH values was measured according to Zielińska et al. (2018) with some modifications. Briefly, each TMP sample was fully dispersed in 10 mM PBS (pH 7.0), then the pH of each solution was adjusted to 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, and 11.0 with 1.0 M HCl or 1.0 M NaOH. The final concentration of each TMP solution at a given pH value was uniformly adjusted to 10.0 mg/mL by using PBS with different pH values, followed by stirring at 500 rpm for 30 min. After that, each solution was centrifuged at 8000 g for 15 min at 4 °C. Protein concentrations of whole solutions and supernatants were measured using the Biuret method. The protein solubility was calculated as follows:
where Ps is the protein concentration of supernatant, and Pw is the protein concentration of the whole solution.
2.8.2. Water holding capacity (WHC) and oil holding capacity (OHC)
The WHC and OHC were determined according to the method of Zielińska et al. (2018) with some modifications.
For WHC, the TMP samples (0.5 g) were dispersed in distilled water (20.0 mL), stirred at 540 rpm using an IKA mechanical mixer for 30 min, and then centrifuged at 8000 g for 15 min at 4 °C. Each precipitate was weighed, and the weight difference was calculated. The results were expressed in grams of water absorbed per gram of sample.
For OHC, the TMP samples (0.5 g) were added to vegetable oil (10.0 mL) and mixed via a vortex mixer (3030A, Scientific Industries, INC., Bohemia) for 30 s and then centrifuged at 8000 g for 15 min at 4 °C. Each precipitate was weighed, and the weight difference was calculated. The results were expressed in grams of oil absorbed per gram of the sample.
2.8.3. Emulsifying properties
The emulsification activity index (EAI) and emulsification stability index (ESI) of each TMP sample were measured according to the method of Chen et al. (2019). Briefly, the emulsions were prepared by adding 9.0 mL of each TMP sample solution (10.0 mg/mL) and 1.0 mL soybean oil into a 50 mL centrifuge tube, followed by homogenization using a homogenizer (IKA, T18 digital Ultra-turrax, Germany) for 2 min at 13,500 rpm. After that, 50 μL of each freshly prepared emulsion was immediately pipetted from the location 0.5 cm away from the bottom of the centrifuge tube and diluted in 4.95 mL of 0.1% (w/v) sodium dodecyl sulfate (SDS) solution. The above dilutions were measured for absorbance at 500 nm via a spectrophotometer (T6, Purkinje General Instrument Co. Ltd., Beijing, China). EAI (m2/g) and ESI (%) were calculated as follows:
where A10 and A0 are the absorbances at 500 nm after 10 min and at time 0, respectively. C is the protein concentration (mg/mL), φ is the volume fraction of the oil phase in the emulsion, and L is the optical path (In this experiment, a colorimetric dish with a light diameter of 1 cm was selected), D is the dilution factor.
2.8.4. Foaming properties
The foaming capacity of each TMP sample was evaluated using a foam analyzer (JPM2012A, Huanqiu Hengda Co., Ltd. Beijing, China). Briefly, 25.0 mL of each TMP solution (0.5 mg/mL) was transferred into a 300 mm height quartz column (inner diameter = 30 mm). Then, nitrogen was injected into each solution at a rate of 0.3 L/min for 1 min. After that, the volume and height of the foam and the reduced volume of each solution were recorded immediately. Finally, after being undisturbed for 900 s, the height of the foam was recorded. Foaming capacity (FC) and Foaming stability (FS) were calculated as follows:
where VF is the volume of the foam after nitrogen injection, VR is the reduced volume of each solution after nitrogen injection, HF is the height of the foam after nitrogen injection, and H900 is the height of the foam after 900 s.
2.9. Statistical analysis
Three independent batches of TMP (replicates) were prepared. For each batch of TMP, measurements of related traits were performed in triplicate. All data were expressed as the mean ± standard deviations (SD). One-way analysis of variance (ANOVA) along with Duncan's test was performed using Statistical software (version 19.0, IBM SPSS Statistics, IBM., Chicago, USA) with a statistical significance level of 0.05.
3. Results and discussion
3.1. Physicochemical properties
3.1.1. Protein yield and basic components of TMP
Protein yield and basic components of extracted TMP are shown in Table 1. The protein yield of the ND group and the FD group were similar (P > 0.05). The MD group had the lowest protein yield (P < 0.05). One possible reason for this was that the heat treatment process of the MD group led to protein denaturation, thereby reducing the protein extraction rate. Moreover, the MD group showed the highest protein content (P < 0.05), whereas the FD group had the lowest protein content (P < 0.05). This phenomenon was probably attributed that the water content (data was not shown) of the MD group was significantly lower than ND group and FD group (P < 0.05), which made the MD group lead to a larger number (quantity) of larvae than the ND group and FD group under the same weight, and subsequently led to the higher amount of chitin in the whole flour of the MD group. Thus, we hypothesized that the TMP extracted from MD group might contain some impurities, and the higher protein content of the MD group was likely due to the presence of more non-protein nitrogen, such as residual chitin (Zhao et al., 2016). Meanwhile, the MD group had the highest fat content (P < 0.05), and due to the hydrophobicity of lipids, the protein-lipid interactions limited the degree of protein dissolution (Lam et al., 2018). In addition, the closer binding of protein and fat caused by thermal denaturation, which was unbeneficial to the subsequent functional application of TMP. Our results indicated that the general defatting method should not efficiently remove the fat/lipid from the TMP, which was similar to some previous work (Purschke et al., 2018; Grossmann et al., 2021; Pasini et al., 2022). Our future work will focus on optimizing the defatting parameters to reduce the fat/lipid content of TMP to the maximum extent.
Table 1.
Protein yield and proximate compositions (dry basis) of TMP extracted from Tenebrio molitor larvae with different pre-extraction treatments.
| ND | MD | FD | |
|---|---|---|---|
| Protein yield (%) | 34.01 ± 0.67A | 12.89 ± 0.43B | 33.82 ± 0.75A |
| Protein content (%) | 84.91 ± 0.91AB | 86.21 ± 1.71A | 83.31 ± 1.21B |
| Fat content (%) | 6.75 ± 0.19B | 9.07 ± 0.95A | 6.43 ± 0.02B |
| Ash content (%) | 3.85 ± 0.08B | 4.76 ± 0.56A | 4.21 ± 0.13AB |
Values are given as means ± SD from triplicate determinations. Different rows (A-B) in the same column indicate significant differences (P < 0.05).
3.1.2. Amino acid composition and protein quality
The protein quality of edible insects is usually judged by the composition of amino acids. In Table 2, all TMP contained all the essential amino acids required by humans (FAO/WHO/UNU, 1985). The sum of the total essential amino acids (EAA) for the ND group was equivalent to the FD group, and each total was higher than human necessity (27.1 g/100g crude protein). However, the sum of EAA for the MD group was lower than the ND and FD groups, and EAA was unavailable in quantities necessary to fulfill human requirements. These results indicate that the analyzed TMP can provide satisfactory amounts of EAA and different pretreatments influence the EAA of each TMP, where the detrimental effect of the MD treatment is apparent. The essential amino acid index (EAAI) can reflect the protein quality. It was observed that the EAAI of the ND and FD groups were similar, but the MD group had the lowest EAAI, which indicates that the protein quality of the MD group was the worst. Similar results have been reported by Brishti et al. (2020), where heating duration had a substantial effect on the percentage of amino acids and caused the depletion of amino acid content. In addition, the total AA for the MD group was lower than the ND and FD groups. Another study found that when compared with full-fat Tenebrio molitor, the defatted Tenebrio molitor contained higher amino acid content, which shows that fat content indirectly affected protein amino acid content (Kim et al., 2020). The fat contents in Table 1 correspond precisely to changes in AA content.
Table 2.
Amino acid profiles and protein quality of TMP extracted from Tenebrio molitor larvae with different pre-extraction treatments.
| Unit (g/100 g protein) | ND | MD | FD | Ref. |
|---|---|---|---|---|
| Essential amino acids (EAA) | ||||
| Histidine (His) | 2.29 | 1.67 | 2.19 | 1.5 |
| Isoleucine (Ile) | 3.90 | 3.19 | 3.81 | 3.0 |
| Leucine (Leu) | 6.50 | 5.08 | 6.41 | 5.9 |
| Lysine (Lys) | 5.24 | 3.31 | 5.02 | 4.5 |
| Methionine + Cysteine (Met + Cys) | 1.70 | 0.51 | 1.84 | 2.2 |
| Phenyl-alanine + Tyrosine (Phe + Tyr) | 8.50 | 6.06 | 8.31 | 3.8 |
| Threonine (Thr) | 3.70 | 2.68 | 3.61 | 2.3 |
| Valine (Val) | 4.65 | 3.72 | 4.39 | 3.9 |
| Sum of EAA | 36.48 | 26.22 | 35.58 | 27.1 |
| Alanine (Ala) | 3.53 | 2.95 | 3.50 | |
| Arginine (Arg) | 4.25 | 3.23 | 4.19 | |
| Aspartic acid (Asp) | 8.83 | 6.34 | 8.51 | |
| Glutamic acid (Glu) | 9.70 | 12.0 | 10.4 | |
| Glycine (Gly) | 3.69 | 5.43 | 3.60 | |
| Proline (Pro) | 3.49 | 2.84 | 3.25 | |
| Serine (Ser) | 3.34 | 3.14 | 3.24 | |
| Sum of total AA | 73.31 | 62.15 | 72.27 | |
| EAAI | 1.15 | 1.10 | 1.14 | |
Reference from FAO/WHO/UNU (1985).
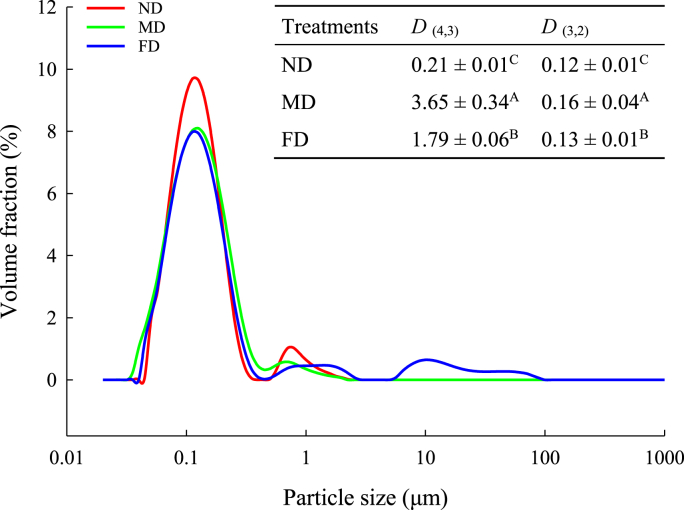
3.1.3. Particle size
The TMP particle size distribution is shown in Fig. 2. The particle size distribution range was narrower in the ND group, followed by the FD group, while the MD group had the widest particle size distribution range. This indicates that the MD group had the largest particle diameter, followed by the FD group, and the ND group had the smallest. The possible reason was that the MD group had the highest fat content, and due to the hydrophobicity of lipids, the protein-lipid interactions limited the degree of protein dissolution (Lam et al., 2018). At the same time, the values of D (4, 3) and D (3, 2) in the table are consistent with the figure trends. Compared with the ND group, the particle size of the FD group was larger (P < 0.05), which might be due to the formation of ice crystals during the freeze-drying process leading to particle aggregation and recomposition into larger particles (Yao et al., 2016). In addition, the MD group had the largest particle size, which was probably caused by thermal denaturation during drying. In conclusion, the results suggest that the particle size of the ND group was the most minor and most uniform. Moreover, when combined with the color results, the ND group had the highest L*-value, indicating that the small particle size allowed a larger surface area to reflect more light.
Fig. 2.
Particle size distribution of TMP extracted from Tenebrio molitor larvae with different pre-extraction treatments.
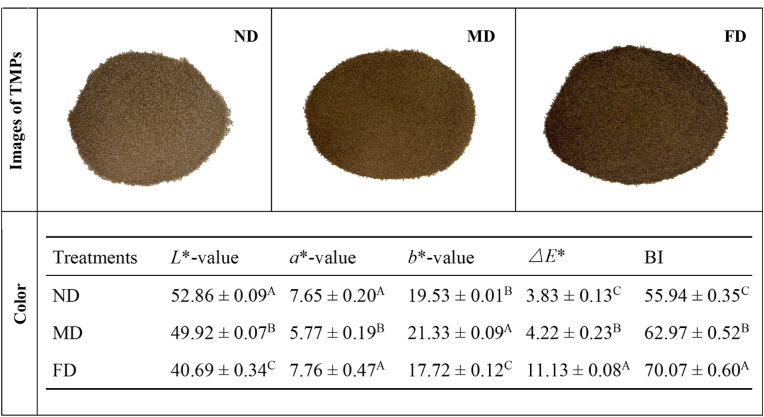
3.1.4. Color
The color properties of proteins are determined by factors such as the intrinsic properties of the protein, the degree of pigment contamination and particle size. As pictured in Fig. 3, all TMP display a brown color. The ND group had the lightest color, followed by the MD group, and the FD group was the darkest. This pattern is consistent with the L*-values in the figure's table. The highest L*-value held by the ND group may be related to the smallest particle size, while the FD group's lowest L*-value might be associated with the maintenance of a low temperature and vacuum combined with long processing times of freeze drying. Yi et al. (2017) indicated that brown color formation took place during protein extraction processing of edible insects, which was most likely due to enzymatic browning reactions. Lenaerts et al. (2018) also suggested that freeze drying could not effectively destroy the enzymes involved in browning reactions and accelerate the non-enzymatic browning reactions, leading to a lower L*-value of edible insect proteins. Moreover, there were no significant differences in the a*-value between the ND and FD groups (P > 0.05), while the MD group had the smallest a*-value (P < 0.05). Meanwhile, compared with the ND group and FD group, the MD group had the largest b*-value. The possible reason might be oxidative reactions within the MD group during the drying process heating conditions forming brown pigments (Brishti et al., 2020). The ΔE*-value represents the total color difference between samples of different pretreatment methods. The parameter is used as a color quality indicator, and the authors consider color differences below 1.5 to be invisible, while values above 6.0 are clearly visible (Lenaerts et al., 2018). The ΔE*-value indicates significant variations for the FD group compared to the MD and ND groups (P < 0.05), which is consistent with the observable difference in the images. In addition, compared with the ND group, the ΔE*-value of the MD group was still significantly different (P < 0.05). The browning index (BI) data displayed in Fig. 3, shows similar variation. Compared with the MD group, the BI value of the ND group remained the lowest (P < 0.05). In contrast, the FD group retained the highest BI value (P < 0.05). Azzollini et al. (2016) indicated that low temperatures and long duration times of freeze drying could not efficiently inhibit the enzymatic browning and subsequently increase the browning index of edible insect proteins. Lenaerts et al. (2018) also found that when compared with freeze drying, microwave drying could partially inactivate the polyphenol oxidase and then prevent the enzymatic browning to some extent. In a word, our results indicated that directly grinding the non-dried edible insects after slaughtering with liquid nitrogen and following with protein extraction process could effectively inhibit the brown color formation during protein isolation.
Fig. 3.
The appearance and color parameters of TMP extracted from Tenebrio molitor larvae with different pre-extraction treatments. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. The structural properties of TMP
3.2.1. SDS-PAGE
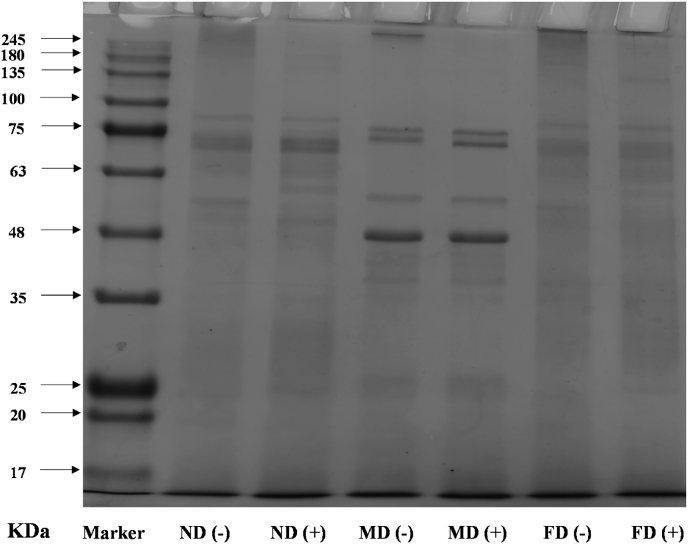
The protein band patterns of SDS-PAGE showed different patterns relating to the pre-treatment methods of TMP (Fig. 4). The overall intensity and pattern of the protein bands varied. Among them, the ND and FD groups had similar band patterns, while the MD group displayed noticeable differences, indicating that the pre-treatment methods changed the molecular weight of the protein, and that MD might have a more significant effect on the molecular weight. Combined with band strength, compared with the ND and FD groups, under non-reducing conditions, the band strength was weakest in the MD group, and the ND and FD groups were similar. These results make sense due to the lower temperature of the FD group drying process and the ND group was not followed by an excessive drying process. Although the MD group drying process took a short time, the temperature was very high, causing severe protein denaturation, aggregation, and cross-linking, thereby reducing the protein solubility and resulting in weaker SDS-PAGE band intensity. This observation is consistent with our solubility results. After adding a reducing agent to destroy the intramolecular disulfide bonds and expand the protein molecules (Timilsena et al., 2016), the band with the highest molecular weight in the MD group spectrum disappeared, and the band strength in the lower molecular weight range increased. However, there was little difference in the banding between the ND group and FD groups regardless of added reducing agent.
Fig. 4.
SDS-PAGE patterns of TMP extracted from Tenebrio molitor larvae with different pre-extraction treatments. (−) represents the electrophoresis samples prepared without β-mercaptoethanol. (+) represents the electrophoresis samples prepared with β-mercaptoethanol.
3.2.2. Sulfhydryl and disulfide bond contents
Sulfhydryl content is an essential conformational aspect in many proteins, which affects the functional changes of proteins. Some processing methods, such as heating and high pressure, might cause changes and destruction of sulfhydryl content, which can significantly affect the functional properties of proteins (Lin et al., 2020). The total sulfhydryl, free sulfhydryl, and disulfide bond content of samples are shown in Table 3. The ND group had the highest value of total sulfhydryl content, followed by the FD group, and the MD group had the lowest total sulfhydryl content (P < 0.05). The likely reason is that the MD group was dried at a higher temperature and lost more sulfhydryl groups than the other groups. Meanwhile, low temperature and low drying pressure during the FD process caused different degrees of protein denaturation (Wang, 2000). Therefore, the content of total sulfhydryl groups in the FD group was also lower than within the ND group (P < 0.05). Free sulfhydryl groups had a similar pattern to total sulfhydryl groups. Compared with the FD group, the MD group had lower free sulfhydryl content (P < 0.05). We hypothesized that the possible reason for this was the resulting protein aggregation due to the high temperature of the MD process (Shen et al., 2020). In addition, a study found that the content of sulfhydryl groups in protein was positively correlated with EAI (Deng et al., 2011). Therefore, compared with the MD and FD groups, the ND group had better emulsification properties, which might be related to the enhanced ability of the ND group to form covalent bonds.
Table 3.
Sulfhydryl contents, disulfide bond contents and surface hydrophobicity of TMP extracted from Tenebrio molitor larvae with different pre-extraction treatments.
| Treatments | Total sulfhydryl (μmol/g) | Free sulfhydryl (μmol/g) | Disulfide bond (μmol/g) | Surface hydrophobicity (μg) |
|---|---|---|---|---|
| ND | 41.34 ± 0.11A | 39.16 ± 0.21A | 3.06 ± 0.35C | 34.96 ± 0.51A |
| MD | 27.40 ± 0.18C | 26.32 ± 0.44C | 5.21 ± 0.16A | 18.68 ± 1.18C |
| FD | 30.71 ± 0.12B | 28.11 ± 0.23B | 3.88 ± 0.02B | 30.33 ± 2.24B |
Values are given as means ± SD from triplicate determinations. Different letters (A-C) in the same column indicate significant differences (P < 0.05).
As shown in Table 3, compared with ND and FD groups, the MD group had the highest disulfide bond contents (P < 0.05). This phenomenon was mainly attributed to a higher temperature of the MD process during drying treatment, which significantly promoted the formation of disulfide bonds. Lin et al. (2020) indicated that the higher drying temperature could enhance the extent of protein denaturation, which subsequently resulted in increased disulfide bond contents. Moreover, the low temperature or freezing stresses could also promote the occurrence of protein denaturation to different extents, which led to obviously higher disulfide bond contents of the FD group than ND group (P < 0.05). The above results were also verified by the changes of SDS-PAGE patterns of TMP samples. Shimada and Cheftel (1988) indicated that the protein aggregation was directly induced by means of SH/SS interchange reactions and/or hydrophobic interchange reactions, which resulted in the different changes of disulfide bond contents of proteins with different pre-treatment parameters, especially for various heating temperatures. Thus, our results indicated that the TMP sample extracted from the ND group had the lowest degree of protein denaturation, which could subsequently render the superior functional properties.
3.2.3. Surface hydrophobicity
Surface hydrophobicity is a crucial protein property that could be used to evaluate changes in protein conformation, and is often related to protein functional properties, such as solubility (Cui et al., 2019). Surface hydrophobicity results are shown in Table 3. The surface hydrophobicity of the ND group was the highest, followed by the FD group, while the MD group had the lowest surface hydrophobicity (P < 0.05). In combination with the solubility results, it was found that the solubility of TMP was lower under neutral conditions, which indicated that these proteins had poor hydrophilicity and the surfaces of the protein powders were more hydrophobic. However, the surface hydrophobicity of the MD and FD groups were lower than that of the ND group (P < 0.05). The possible reason for this was that the drying process caused the protein to polymerize, and the hydrophobic area was buried within the interior of the protein, resulting in lower surface hydrophobicity (Azagoh et al., 2016).
3.2.4. FTIR spectroscopy analysis
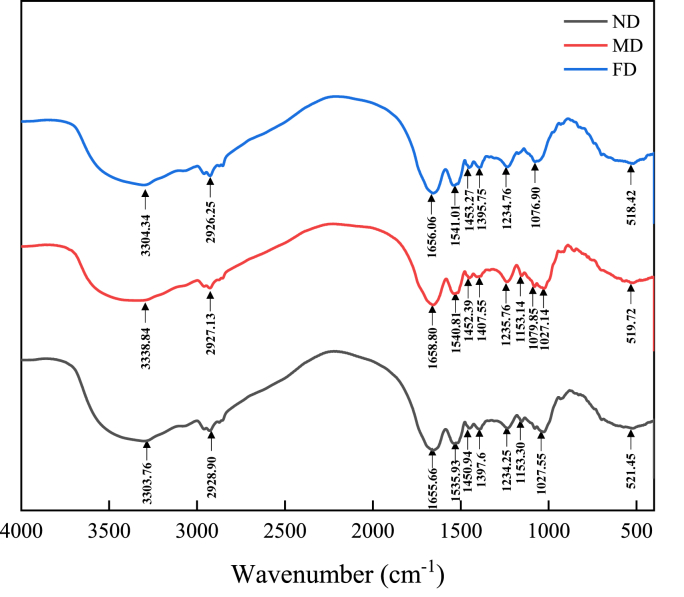
The use of FTIR spectroscopy to evaluate the effects of different pretreatment methods on the secondary structure of the TMP was shown in Fig. 5. All samples had three groups of characteristic absorption bands: amide Ⅰ band, amide Ⅱ band and amide III band, with wave numbers corresponding to 1600–1700 cm−1, 1530–1550 cm−1 and 1260–1300 cm−1respectively (Li et al., 2020). At amide I (Fig. 5), the peak shifted from 1655 cm−1(FD group) to 1658 cm−1(MD group), which indicated that the content of α-helix and the random coil was decreased, and the content of β-sheet was increased. As shown in Table 4, the α-helical content of the ND group and the FD group was 14.74% and 14.42% (P > 0.05), while the MD group was 13.10% (P < 0.05). At the same time, the content of the β-sheet is positively correlated with the α-helical content. Some studies found that the secondary structure of a protein was determined by electrostatic interactions and hydrogen bonds between amino acids (Niu et al., 2019), and when the α-helical structure was denatured by heat, freezing, or oxidation, α-helical structures usually turned into β-sheet (Guo et al., 2019). This result suggests that the MD group has weakened electrostatic interactions, and the stable hydrogen bonds due to heating destroyed the secondary structure of the protein and transformed the α-helical structures into β-sheet. In addition, there was no significant difference in the secondary structure between the ND group and the FD group (P > 0.05).
Fig. 5.
FTIR spectroscopy of TMP extracted from Tenebrio molitor larvae with different pre-extraction treatments.
Table 4.
Percentage of secondary structures of TMP extracted from Tenebrio molitor larvae with different pre-extraction treatments.
| Treatments | α-helix (%) | β-sheet (%) | β-turns (%) | Random coil (%) |
|---|---|---|---|---|
| ND | 14.74 ± 0.20A | 34.60 ± 0.12B | 28.56 ± 0.51A | 25.23 ± 0.35A |
| MD | 13.10 ± 0.43B | 43.03 ± 0.50A | 23.16 ± 0.10B | 21.50 ± 0.14B |
| FD | 14.42 ± 0.35A | 35.90 ± 0.33B | 28.90 ± 0.11A | 25.80 ± 0.40A |
Values are given as means ± SD from triplicate determinations. Different letters (A-B) in the same column indicate significant differences (P < 0.05).
3.2.5. Intrinsic fluorescence analysis
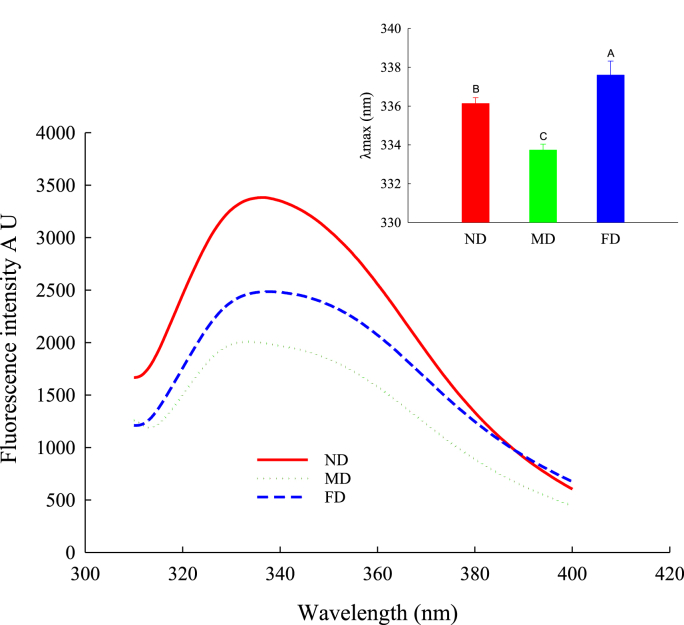
The intrinsic fluorescence intensity of protein is particularly sensitive to the polarity of its microenvironment, so it is widely used to monitor changes in the tertiary structure of proteins (Cao et al., 2016). As shown in Fig. 6, the peak intensity of TMP in the fluorescence spectrum is located at 340 nm, which is consistent with the emission spectra of tryptophan. Compared with the ND and FD groups, the fluorescence spectrum of the MD group happened to be blue-shifted, and the fluorescence intensity was the weakest (P < 0.05). Moreover, the fluorescence peaks of samples treated with ND and FD were similar, but the ND group had more vigorous fluorescence intensity (P < 0.05). A decrease in fluorescence intensity and a redshift of the spectrum may indicate the denaturation of protein and a modification of the environment of a protein (Lefevre et al., 2007). Therefore, our results suggest that the MD group had tryptophan located in a relatively non-polar microenvironment. On the other hand, compared to the ND group, the MD and FD groups had significantly reduced fluorescence intensity, which could be due to the aggregation of proteins during the drying process, the buried amino acids with inherent fluorescent properties, or fluorescence quenching due to oxidation of exposed amino acids (Jiang et al., 2016; Cao et al., 2016).
Fig. 6.
Intrinsic fluorescence of TMP extracted from Tenebrio molitor larvae with different pre-extraction treatments.
3.3. The functional properties of TMP
3.3.1. Assessment of pH-dependent protein solubility
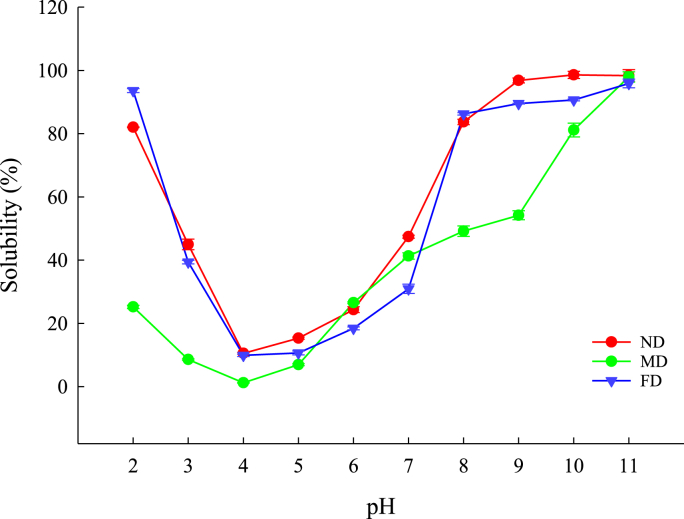
The solubility of proteins is significant when designing methods for extracting proteins and evaluating their functional properties. As shown in Fig. 7, the results show that the solubility of TMP is significantly dependent on pH. The protein solubility of all samples was the smallest at pH 4, with values of 10.47% for the ND group, 1.16% for the MD group, and 9.55% for the FD group. The solubility of the ND and FD groups increased significantly at pH 8, while the MD group solubility increased significantly at pH 9. The highest solubility among samples was apparent at pH 11, but high solubility values were also evident at pH 2 and 3. These results correspond well with Zielińska et al. (2018) in similar assay conditions but for Tenebrio molitor protein extraction. The solubility trends of the ND and FD groups were similar, but the solubility of the ND group was better. Compared with the ND and FD groups, the MD group had the worst solubility. Another study reported that solubility was usually affected by its hydrophobic/hydrophilic balance, which depended on the amino acid composition of the protein and the degree of denaturation (Santos et al., 2011). Therefore, combined with the results of surface hydrophobicity and amino acids, it was found that the MD group's poor solubility of protein was due to fewer hydrophobic residues, increased charge, electrostatic repulsion, and ionic hydration at different pH values (Lin et al., 2020).
Fig. 7.
The solubility of TMP extracted from Tenebrio molitor larvae with different pre-extraction treatments as a function of various pH values.
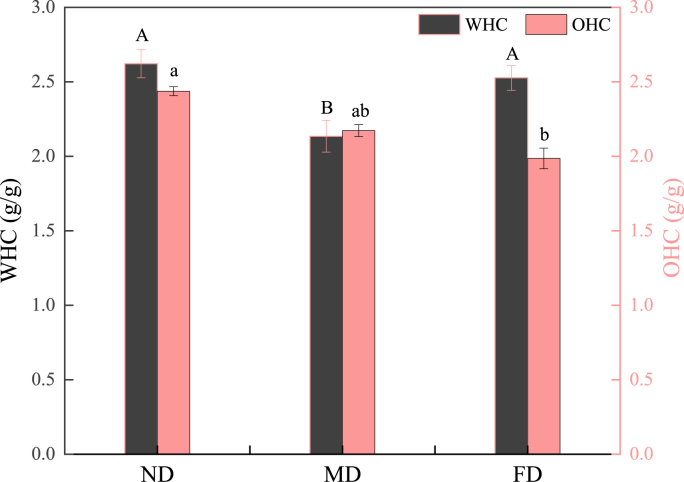
3.3.2. WHC and OHC
WHC is a crucial attribute denoting water retention function, swelling, dissolution, and gel properties of protein. Another key attribute is OHC which indicates the ability of the protein to absorb and retain fat. Both of these attributes are vital in evaluating the texture and quality of food (Foegeding and Davis, 2011). As shown in Fig. 8, the WHC of the ND and FD groups had no significant differences (P > 0.05), and the MD group had the lowest WHC value (P < 0.05). The higher WHC of the ND group and the FD group might be due to lower loss of soluble protein and less protein denaturation, while the lower WHC of the MD group was attributed to the external moisture-resistant skin formed during the drying process (Lin et al., 2020). There was no significant difference between the OHC of the MD group and the FD group (P > 0.05), while the ND group had the highest OHC (P < 0.05). The surface hydrophobicity of the ND group was significantly higher than that of the other two pretreatment methods (Table 3), which made it have the highest oil absorption capacity. At the same time, the smaller particle size (Fig. 2) and larger surface area might explain why the amount of fat adsorption was the highest. The poor OHC of the MD group and the FD group might be caused by the conformational characteristics, surface hydrophobicity, lipophilic groups and denatured factors of these proteins.
Fig. 8.
Water holding capacity (WHC) and oil holding capacity (OHC) of TMP extracted from Tenebrio molitor larvae with different pre-extraction treatments. The different uppercase letters (A–B) indicate significant differences of WHC among different groups, and the different lowercase letters (a–b) indicate significant differences of OHC among different groups (P < 0.05).
3.3.3. Emulsifying properties
The oil-water interface is dominated by hydrophobic interactions, and the exposure of non-polar hydrophobic residues at the interface significantly affects emulsification performance. Higher surface hydrophobicity could make an emulsifier combine with oil droplets more strongly, leading to better emulsifying properties of the protein (Gong et al., 2016). As shown in Table 5, the ND group has the highest EAI, followed by the FD group, and the MD group has the lowest value (P < 0.05). The lower emulsifying properties of the MD group may be related to its lower solubility. Although the thermal denaturation of the MD group was greater than that of the FD group, both drying methods had a negative effect on ESI. The ND group had the highest ESI (P < 0.05). Hu et al. (2010) observed that the ESI and surface hydrophobicity of soy protein isolate dried in a vacuum oven were the lowest at 60 °C, which confirmed the possibility of protein aggregation, making it difficult for the protein to form a stable film around oil droplets over time. These results are similar to the FD and MD group results in this paper. This suggests that the drying process caused protein aggregation leading to reduced emulsification performance. Therefore, with fewer pretreatment steps, the emulsification performance was better.
Table 5.
Emulsifying and foaming properties of TMP extracted from Tenebrio molitor larvae with different pretreatment methods.
| Treatments | EAI (m2/g) | ESI (%) | Foaming capacity (%) | Foam stability (mm) |
|---|---|---|---|---|
| ND | 45.10 ± 0.46A | 57.85 ± 1.49A | 4577.33 ± 7.02A | 166.20 ± 1.73A |
| MD | 22.10 ± 0.53C | 40.78 ± 2.71B | 3816.53 ± 8.18C | 115.34 ± 0.57C |
| FD | 25.62 ± 0.56B | 39.17 ± 2.05B | 4296.78 ± 7.93B | 138.67 ± 2.08B |
Values are given as means ± SD from triplicate determinations. Different letters (A-C) in the same column indicate significant differences (P < 0.05).
3.3.4. Foaming properties
The FC and FS of protein are generally affected by many factors, such as protein conformation, solubility, available hydrophobic sites and concentration. Therefore, different pretreatment methods changed the foaming properties of the TMP. Table 5 shows the influence of different pretreatment methods on the foaming properties of TMP. Compared with the MD and FD groups, the ND group had the highest FC and FS (P < 0.05). Proteins with ideal foaming properties generally have high surface hydrophobicity and good solubility (Lin et al., 2020). Therefore, in Table 3 and Fig. 7, we found that the ND group has the highest surface hydrophobicity and solubility, so the samples had the best foaming performance. However, as described above, the drying methods affected the structure and hydrophobicity of the MD and FD groups, which in turn affected their adsorption rates at the air-water interface and made their foaming performances worse.
4. Conclusion
The results indicated that the TMP extracted from the ND group showed higher essential/total amino acid content, total/free sulfhydryl content, surface hydrophobicity, solubility, WHC, OHC, EAI, ESI, FC, and FS than those obtained from either MD or FD group. Moreover, compared with conventional drying treatments, non-drying treatment had less impactful changes to the structural properties of TMP, which would be generally associated with heat denaturation. In addition, TMP extracted from the MD group had the lowest protein yield and functional properties, as well as the highest degree of protein denaturation (implied by the results of SDS-PAGE). Therefore, it can be concluded that our proposed pre-extraction treatment (non-drying treatment) for TMP extraction from Tenebrio molitor larvae could obviously improve the functional properties of TMP with less protein denaturation and energy consumption. Our future work will focus on the comparative study of nutritional and digestive characteristics of TMP extracted from Tenebrio molitor larvaes subjected to different pre-extraction treatments.
CRediT authorship contribution statement
Fengxue Zhang: Methodology, Investigation, Writing – original draft. Yining Xu: Software, Investigation, Validation. Baohua Kong: Data curation, Methodology. Qian Chen: Investigation, Data curation. Fangda Sun: Visualization, Resources. Hongwei Zhang: Investigation, Methodology. Qian Liu: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the Natural Science Funds for Distinguished Young Scholars of Heilongjiang Province (Grant No. JQ2021C003), National Natural Science Foundation of China (Grant No. 32172233), and Foundation of Central Support for the Reform and Development of Local Universities in Heilongjiang Province (Excellent Young Talents Project) (Grant No. 2020YQ15).
Editor name: Dr. Xing Chen
References
- Azagoh C., Ducept F., Garcia R., Rakotozafy L., Cuvelier M.E., Keller S., Lewandowski R., Mezdour S. Extraction and physicochemical characterization of Tenebrio molitor proteins. Food Res. Int. 2016;88:24–31. doi: 10.1016/j.foodres.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Azzollini D., Derossi A., Severini C. Understanding the drying kinetic and hygroscopic behaviour of larvae of yellow mealworm (Tenebrio molitor) and the effects on their quality. J. Insects Food Feed. 2016;2:233–243. https://doi:10.3920/jiff2016.0001 [Google Scholar]
- Brishti F.H., Chay S.Y., Muhammad K., Ismail-Fitry M.R., Zarei M., Karthikeyan S. Effects of drying techniques on the physicochemical, functional, thermal, structural and rheological properties of mung bean (Vigna radiata) protein isolate powder. Food Res. Int. 2020;138 doi: 10.1016/j.foodres.2020.109783. [DOI] [PubMed] [Google Scholar]
- Cao Y., True A.D., Chen J., Xiong Y.L. Dual role (anti-and pro-oxidant) of gallic acid in mediating myofibrillar protein gelation and gel in vitro digestion. J. Agric. Food Chem. 2016;64:3054–3061. doi: 10.1021/acs.jafc.6b00314. [DOI] [PubMed] [Google Scholar]
- Chelh I., Gatellier P., Santé-Lhoutellier V. Technical note: a simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006;74:681–683. doi: 10.1016/j.meatsci.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., Jiang S., Chen Q., Liu Q., Kong B.H. Antioxidant activities and emulsifying properties of porcine plasma protein hydrolysates modified by oxidized tannic acid and oxidized chlorogenic acid. Process Biochem. 2019;79:105–113. doi: 10.1016/j.procbio.2018.12.026. [DOI] [Google Scholar]
- Cui Y., Li X., Liu M., Liu X., Duan X. Role of polysaccharide conjugation in physicochemical and emulsifying properties of egg phosvitin and the calcium binding capacity of its phosphopeptides. Food Funct. 2019;10:1808–1815. doi: 10.1039/C8FO02464B. [DOI] [PubMed] [Google Scholar]
- Deng Q., Wang L., Wei F., Xie B., Huang F., Huang W., Shi J., Huang Q., Tian B., Xue S. Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem. 2011;124:1458–1465. doi: 10.1016/j.foodchem.2010.07.108. [DOI] [Google Scholar]
- FAO/WHO/UNU . 1985. Energy and Protein Requirements: Report of a Joint Fao/who/unu Expert Consultation; p. 206. Geneva, Switzerland. [PubMed] [Google Scholar]
- Feng Y.Y., Ma X.L., Kong B.H., Chen Q., Liu Q. Ethanol induced changes in structural, morphological, and functional properties of whey proteins isolates: influence of ethanol concentration. Food Hydrocolloids. 2021;111 doi: 10.1016/j.foodhyd.2020.106379. [DOI] [Google Scholar]
- Foegeding E.A., Davis J.P. Food protein functionality: a comprehensive approach. Food Hydrocolloids. 2011;25:1853–1864. doi: 10.1016/j.foodhyd.2011.05.008. [DOI] [Google Scholar]
- Gao W.H., Huang Y.P., Zeng X.A., Brennan M.A. Effect of soluble soybean polysaccharides on freeze-denaturation and structure of myofibrillar protein of bighead carp surimi with liquid nitrogen freezing. Int. J. Biol. Macromol. 2019;135:839–844. doi: 10.1016/j.ijbiomac.2019.05.186. [DOI] [PubMed] [Google Scholar]
- Gong K.J., Shi A.M., Liu H.Z., Liu L., Hu H., Adhikari B., Wang Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016;170:33–40. doi: 10.1016/j.jfoodeng.2015.09.011. [DOI] [Google Scholar]
- Gould J., Wolf B. Interfacial and emulsifying properties of mealworm protein at the oil/water interface. Food Hydrocolloids. 2018;77:57–65. doi: 10.1016/j.foodhyd.2017.09.018. [DOI] [Google Scholar]
- Gravel A., Doyen A. The use of edible insect proteins in food: challenges and issues related to their functional properties. Innovat. Food Sci. Emerg. Technol. 2020;59 doi: 10.1016/j.ifset.2019.102272. [DOI] [Google Scholar]
- Grossmann K.K., Merz M., Appel D., De Araujo M.M., Fischer L. New insights into the flavoring potential of cricket (Acheta domesticus) and mealworm (Tenebrio molitor) protein hydrolysates and their Maillard products. Food Chem. 2021;364 doi: 10.1016/j.foodchem.2021.130336. [DOI] [PubMed] [Google Scholar]
- Guo X.J., Shi L., Xiong S.B., Hu Y., You J., Huang Q.L., Yin T. Gelling properties of vacuum-freeze dried surimi powder as influenced by heating method and microbial transglutaminase. LWT--Food Sci. Technol. 2019;99:105–111. doi: 10.1016/j.lwt.2018.09.050. [DOI] [Google Scholar]
- Hu X.Z., Cheng Y.Q., Fan J.F., Lu Z.H., Yamaki K., Li L.T. Effects of drying method on physicochemical and functional properties of soy protein isolates. J. Food Process. Preserv. 2010;34:520–540. https://10.1111/j.1745-4549.2008.00357.x [Google Scholar]
- Jiang S., Zhao S.C., Jia X.W., Zhang H., Liu Q., Kong B.H. Thermal gelling properties, structural properties and mechanism of myofibrillar protein including thermo-reversible and thermo-irreversible curdlan gels. Food Chem. 2020;311 doi: 10.1016/j.foodchem.2019.126018. [DOI] [PubMed] [Google Scholar]
- Jiang W.X., He Y.F., Xiong S.B., Liu Y.M., Yin T., Hu Y., You J. Effect of mild ozone oxidation on structural changes of silver carp (hypophthalmichthys molitrix) myosin. Food Bioprocess Technol. 2016;10:370–378. doi: 10.1007/s11947-016-1828-5. [DOI] [Google Scholar]
- Kim H.W., Setyabrata D., Lee Y.J., Jones O.G., Kim Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innovat. Food Sci. Emerg. Technol. 2016;38:116–123. doi: 10.1016/j.ifset.2016.09.023. [DOI] [Google Scholar]
- Kim T.K., Yong H.I., Chun H.H., Lee M.A., Kim Y.B. Changes of amino acid composition and protein technical functionality of edible insects by extracting steps. J. Asia Pac. Entomol. 2020;23:298–305. doi: 10.1016/j.aspen.2019.12.017. [DOI] [Google Scholar]
- Kröncke N., Böschen V., Woyzichovski J., Demtröder S., Benning R. Comparison of suitable drying processes for mealworms (Tenebrio molitor) Innovat. Food Sci. Emerg. Technol. 2018;50:20–25. doi: 10.1016/j.ifset.2018.10.009. [DOI] [Google Scholar]
- Lam A.C.Y., Can Karaca A., Tyler R.T., Nickerson M.T. Pea protein isolates: structure, extraction, and functionality. Food Res. Int. 2018;34:126–147. doi: 10.1080/87559129.2016.1242135. [DOI] [Google Scholar]
- Lefevre F., Fauconneau B., Thompson J.W., Gill T.A. Thermal denaturation and aggregation properties of Atlantic salmon myofibrils and myosin from white and red muscles. J. Agric. Food Chem. 2007;55:4761–4770. doi: 10.1021/jf063045d. [DOI] [PubMed] [Google Scholar]
- Lenaerts S., van der Borght M., Callens A., Campenhout L.V. Suitability of microwave drying for mealworms (Tenebrio molitor) as alternative to freeze drying: impact on nutritional quality and colour. Food Chem. 2018;254:129–136. doi: 10.1016/j.foodchem.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Leni G., Caligiani A., Sforza S. Killing method affects the browning and the quality of the protein fraction of Black Soldier Fly (Hermetia illucens) prepupae: a metabolomics and proteomic insight. Food Res. Int. 2019;115:116–125. doi: 10.1016/j.foodres.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Li Y.H., Cheng Y., Zhang Z.L., Wang Y., Mintah B.K., Dabbour M., Jiang H., He R.H., Ma H.L. Modification of rapeseed protein by ultrasound-assisted pH shift treatment: ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrason. Sonochem. 2020;69 doi: 10.1016/j.ultsonch.2020.105240. [DOI] [PubMed] [Google Scholar]
- Lin N., Liu B.L., Liu Z.D., Qi T. Effects of different drying methods on the structures and functional properties of phosphorylated Antarctic krill protein. J. Food Sci. 2020;85:3690–3699. doi: 10.1111/1750-3841.15503. [DOI] [PubMed] [Google Scholar]
- Liu Q., Geng R., Zhao J.Y., Chen Q., Kong B.H. Structural and gel textural properties of soy protein isolate when subjected to extreme acid pH-shifting and mild heating processes. J. Agric. Food Chem. 2015;63:4853–4861. doi: 10.1021/acs.jafc.5b01331. [DOI] [PubMed] [Google Scholar]
- Liu Q., Kong B.H., Han J.C., Sun C.Y., Li P.J. Structure and antioxidant activity of whey protein isolate conjugated with glucose via the Maillard reaction under dry-heating conditions. Food Struct-Neth. 2014;1:145–154. doi: 10.1016/j.foostr.2013.11.004. [DOI] [Google Scholar]
- Megido R.C., Poelaert C., Ernens E.M., Liotta M., Blecker C., Danthine S., Tyteca E., Haubruge E., Alabi T., Bindelle J., Francis F. Effect of household cooking techniques on the microbiological load and the nutritional quality of mealworms (Tenebrio molitor L. 1758) Food Res. Int. 2018;106:503–508. doi: 10.1016/j.foodres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Melgar-Lalanne G., Hernández-Álvarez A.J., Salinas-Castro A. Edible insects processing: traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 2019;18:1166–1191. doi: 10.1111/1541-4337.12463. [DOI] [PubMed] [Google Scholar]
- Mohapatra D., Bira Z.M., Kerry J.P., Frías J.M., Rodrigues F.A. Postharvest hardness and color evolution of white button mushrooms (Agaricus bisporus) J. Food Sci. 2010;75:E146–E152. doi: 10.1111/j.1750-3841.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- Niu J.Z., Zhao B.F., Guo X.J., Yin T. Effects of vacuum freeze-drying and vacuum spray-drying on biochemical properties and functionalities of myofibrillar proteins from silver carp. J. Food Qual. 2019;1–8 doi: 10.1155/2019/9457835. [DOI] [Google Scholar]
- Pasini G., Cullere M., Vegro M., Simonato B., Zotte A.D. Potentiality of protein fractions from the house cricket (Acheta domesticus) and yellow mealworm (Tenebrio molitor) for pasta formulation. LWT--Food Sci. Technol. 2022;164 doi: 10.1016/j.lwt.2022.113638. [DOI] [Google Scholar]
- Payne C.L.R., Dobermann D., Forkes A., House J., Josephs J., McBride A., Müller A., Quilliam R.S., Soares S. Insects as food and feed: european perspectives on recent research and future priorities. J. Insects Food Feed. 2016;2:269–276. doi: 10.17863/CAM.483. [DOI] [Google Scholar]
- Purschke B., Brüggen H., Scheibelberger R., Jäger H. Effect of pre-treatment and drying method on physicochemical properties and dry fractionation behaviour of mealworm larvae (Tenebrio molitor L.) Eur. Food Res. Technol. 2018;244:269–280. doi: 10.1007/s00217-017-2953-8. [DOI] [Google Scholar]
- Sánchez-Muros M.J., Barroso F.G., Manzano-Agugliaro F. Insect meal as renewable source of food for animal feeding: a review. J. Clean. Prod. 2014;65:16–27. doi: 10.1016/j.jclepro.2013.11.068. [DOI] [Google Scholar]
- Santos S.D.A.D., Martins V.G., Salas-Mellado M., Prentice C. Evaluation of functional properties in protein hydrolysates from Bluewing Searobin (Prionotus punctatus) obtained with different microbial enzymes. Food Bioprocess Technol. 2011;4:1399–1406. doi: 10.1007/s11947-009-0301-0. [DOI] [Google Scholar]
- Shen L., Li J.L., Lv L.S., Zhang L., Bai R., Zheng T.S., Zhang Q.T. Comparison of functional and structural properties of ginkgo seed protein dried by spray and freeze process. J. Food Sci. Technol. 2020;58:1–11. doi: 10.1007/s13197-020-04527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Cheftel J.C. Determination of sulfhydryl groups and disulfide bonds in heat-induced gels of soy protein isolate. J. Agric. Food Chem. 1988;36:147–153. doi: 10.1021/jf00079a038. [DOI] [Google Scholar]
- Sun-Waterhouse D., Waterhouse G.I.N., You L., Zhang J., Liu Y., Ma L., Gao J., Dong Y. Transforming insect biomass into consumer wellness foods: a review. Food Res. Int. 2016;89:129–151. doi: 10.1016/j.foodres.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Timilsena Y.P., Adhikari R., Barrow C.J., Adhikari B. Physicochemical and functional properties of protein isolate produced from Australian chia seeds. Food Chem. 2016;212:648–656. doi: 10.1016/j.foodchem.2016.06.017. [DOI] [PubMed] [Google Scholar]
- van Huis A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013;58:563–583. doi: 10.1146/annurev-ento-120811-153704. [DOI] [PubMed] [Google Scholar]
- Vandeweyer D., Lenaerts S., Callens A., Van Campenhout L. Effect of blanching followed by refrigerated storage or industrial microwave drying on the microbial load of yellow mealworm larvae (Tenebrio molitor) Food Control. 2017;71:311–314. doi: 10.1016/j.foodcont.2016.07.011. [DOI] [Google Scholar]
- Wang W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000;203:1–60. doi: 10.1016/S0378-5173(00)00423-3. [DOI] [PubMed] [Google Scholar]
- Wessels M.L.J., Azzollini D., Fogliano V. Frozen storage of lesser mealworm larvae (Alphitobius diaperinus) changes chemical properties and functionalities of the derived ingredients. Food Chem. 2020;320 doi: 10.1016/j.foodchem.2020.126649. [DOI] [PubMed] [Google Scholar]
- Yang Z.M., Liu S.C., Sun Q.X., Zheng O.Y., Wei S., Xia Q.Y., Ji H.W., Deng C.J., Hao J.M., Xu J. Insight into muscle quality of golden pompano (Trachinotus ovatus) frozen with liquid nitrogen at different temperatures. Food Chem. 2022;374 doi: 10.1016/j.foodchem.2021.131737. [DOI] [PubMed] [Google Scholar]
- Yao Y.P., Zhao G.Z., Yan Y.Y., Chen C., Sun C., Zou X.Q., Jin Q.Z., Wang X.G. Effects of freeze drying and spray drying on the microstructure and composition of milk fat globules. RSC Adv. 2016;6:2520–2529. doi: 10.1039/C5RA22323G. [DOI] [Google Scholar]
- Yen A.L. Insects as food and feed in the Asia Pacific region: current perspectives and future directions. J. Insects Food Feed. 2015;1:33–55. doi: 10.3920/JIFF2014.0017. [DOI] [Google Scholar]
- Yi L.Y., Boekel M.A.J.S.V., Lakemond C.M.M. Extracting Tenebrio molitor protein while preventing browning: effect of pH and NaCl on protein yield. J. Insects Food Feed. 2017;3:21–31. doi: 10.3920/JIFF2016.0015. [DOI] [Google Scholar]
- Yi L.Y., Lakemond C.M.M., Sagis L.M.C., Eisner-Schadler V., Huis A.V., Boekel M.A.J.S.V. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013;141:3341–3348. doi: 10.1016/j.foodchem.2013.05.115. [DOI] [PubMed] [Google Scholar]
- Zhao X., Vázquez-Gutiérrez J.L., Johansson D.P., Landberg R., Langton M. Yellow mealworm protein for food purposes-extraction and functional properties. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska E., Karaś M., Baraniak B. Comparison of functional properties of edible insects and protein preparations thereof. LWT--Food Sci. Technol. 2018;91:168–174. doi: 10.1016/j.lwt.2018.01.058. [DOI] [Google Scholar]