Abstract
Background
Penile cancer is rare among male malignancies. Various biomarkers have been used to predict the prognosis of cancer, one of which is the neutrophil to lymphocyte ratio (NLR). Therefore, we conducted this systematic review and meta-analysis to evaluate the prognostic value of NLR in penile cancer.
Methods
This review was conducted following the PRISMA guideline. Several databases, including Scopus, Science-direct, and PubMed, were systematically searched. The primary outcomes were lymph node metastasis (LNM), cancer-specific survival (CSS), and overall survival (OS). All statistical analyses were processed using Review Manager (RevMan) version 5.4.
Results
A total of six retrospective studies were included in the analysis. The cut-off values of NLR in the included studies ranged from 2.6 to 3.59. Meta-analysis showed that penile cancer patients with high NLR had worse LNM and CSS based on the univariate analysis (OR 3.56, 95% CI 2.38, 5.32, p < 0.01; HR 4.19, 95% CI 2.19, 8.01, p = 0.0; respectively). Furthermore, the meta-analysis revealed that NLR is an independent predictor of LNM and CSS (OR 6.67, 95% CI 2.44, 18.22, p < 0.01; HR 2.15, 95% CI 1.23, 3.73, p < 0.01; respectively). However, NLR failed to show as independent predictor for OS (HR 1.69,95% CI 0.95,3.00, p = 0.07).
Conclusion
NLR is an independent predictor of LNM and CSS. However, NLR is not proven to be an independent predictor of OS in this study.
Keywords: Penile cancer, Neutrophil to lymphocyte ratio, Overall survival, Cancer-specific survival, Cancer
Highlights
-
•
NLR is an independent predictor of LNM in penile cancer.
-
•
NLR is an independent predictor of CSS in penile cancer.
-
•
This study failed to demonstrate the association of NLR as predictor for OS.
1. Introduction
Penile cancer is rare among male malignancies and causes a substantial psychological effect on the patient. In the United States and Europe, penile cancer only accounts for 0.4–0.6% of malignant diagnoses [1,2]. On the contrary, the Brazilian state of Maranhão has the world's highest incidence of penile cancer (ASR of 6.1 cases per 100,000 people) [3]. Patients with inguinal lymph node involvement have a poor prognosis [4,5]. Predictors of inguinal lymph node metastasis consisted of the pathological stage of the primary tumor, grade, and lymphovascular invasion. Penile cancer is likely incurable once systemic metastasis has occurred. Local lymphatic dissemination to regional lymph nodes in the inguinal area occurs stepwise, with the superficial inguinal lymph nodes usually the first primary points of cancer metastasis [6].
Recent literature highlights that the association between systemic inflammation and tumor development is confirmed in various solid organ cancers [7]. Since complete blood count can signify underlying systemic inflammation, the Neutrophil to Lymphocyte Ratio (NLR) invariably serves as an indicator of tissue inflammation in cancer [8]. The NLR measures the systemic inflammatory and immunological responses. Several studies have evaluated the predictive use of pre-treatment NLR as an independent predictor of overall survival (OS) in patients with penile squamous cell carcinoma and inguinal lymph node. NLR also corresponds to the nodal stage of the tumor. Published data showed a positive association between systemic inflammation and survival/prognosis [[9], [10], [11], [12]]. This systematic review and meta-analysis evaluated the prognostic value of NLR in penile cancer.
2. Methods
2.1. Study design
This systematic review and meta-analysis study was conducted by following the guideline of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [13]. Self-evaluation of this study was assessed and in accordance with AMSTAR 2 criteria [14]. The study protocol was registered in PROSPERO (CRD42022271381) and research registry (reviewregistry1415).
2.2. Systematic Search strategy
A comprehensive online literature search was performed to select the potential studies on PubMed, Scopus, and ScienceDirect library database inception of December 2021. The following keyword was used (“NLR” or “Neutrophils-to-Lymphocyte Ratio” or “neutrophil to lymphocyte ratio”) and (“penile cancer” or “carcinoma of the penis” or “malignancy of penis”). A complete search strategy for included studies is provided in Table 1.
Table 1.
Keywords used as literature search strategy.
| Search-engine | Keywords | Article(n) |
|---|---|---|
| PubMED | (“neutrophil-to-lymphocyte ratio"[All Fields] OR “neutrophil-to-lymphocyte ratio"[All Fields] OR “nlr"[All Fields]) AND (((“penil"[All Fields] OR “penis"[MeSH Terms] OR “penis"[All Fields] OR “penile"[All Fields]) AND (“carcinoma"[MeSH Terms] OR “carcinoma"[All Fields] OR “carcinomas"[All Fields] OR “carcinoma s"[All Fields])) OR (“penile neoplasms"[MeSH Terms] OR (“penile"[All Fields] AND “neoplasms"[All Fields]) OR “penile neoplasms"[All Fields] OR (“penile"[All Fields] AND “cancer"[All Fields]) OR “penile cancer"[All Fields]) OR (“penile neoplasms"[MeSH Terms] OR (“penile"[All Fields] AND “neoplasms"[All Fields]) OR “penile neoplasms"[All Fields] OR (“penis"[All Fields] AND “neoplasms"[All Fields]) OR “penis neoplasms"[All Fields])) | 12 |
| Scopus | TITLE-ABS-KEY ((“neutrophil two lymphocyte ratio” OR “neutrophil-two-lymphocyte ratio” OR nlr) AND (penile AND carcinoma OR penile AND cancer OR penis AND neoplasms)) | 18 |
| Science-direct | (“neutrophil to lymphocyte ratio” OR “neutrophil-to-lymphocyte ratio” OR nlr) AND (penile carcinoma OR penile cancer OR Penis neoplasms) | 92 |
| 122 |
2.3. Eligibility criteria
The study enrolled in this systematic review and meta-analysis should meet these inclusion criteria: (1) studies which compared penile cancer patients with high NLR to patients with low NLR before surgery to determine predictor of oncological and metastatic outcomes; (2) Required data can be extracted; (3) Publication articles were available on PubMed, ScienceDirect, and Scopus database. The studies were excluded following these exclusion criteria: (1) case report, case series, and article review, (2) In vitro research, (3) animal studies, and (4) unpublished article.
2.4. Data extraction and quality assessment
Three investigators (HMS, FH and YPK) independently extracted the following items: study characteristics (authors, years of publication, location of studies, sample size); baseline characteristics of the sample (type of histopathology, age of the samples, modality of treatment, and follow up duration); NLR cut-off values; cut off values methods; outcome (lymph node metastasis (LNM), cancer-specific survival (CSS), and overall survival (OS). The Newcastle-Ottawa Scale (NOS), which includes selection, comparability, and exposure, was used to assess the risk of bias in each enrolled literature [15]. The score classification was described in 0–3 as a low-quality study, while 4–6 as a medium quality study, and 7–9 as a high-quality study. When there were discrepancies between the two investigators, the decision was made after being discussed with the third investigator.
The outcomes of this study were LNM, CSS, and OS. LNM was calculated from the result of the clinicopathology data. Pooled Odd Ratio (OR) was extracted directly if reported in the studies. In survival analysis, the HRs and 95% confidence intervals (CIs) were extracted and used to calculate pooled hazard ratio (HR). I2 statistics were used to assess the heterogeneity among the included studies. If significant heterogeneity existed (I2 >50% and/or p < 0.10), the pooled HRs and 95% CIs were calculated by a random-effect model; otherwise, the fixed-effect model was performed (I2 < 50% and/or p > 0.10). All the analysis was performed with RevMan 5.4 for windows.
3. Results
3.1. Systematic Search results
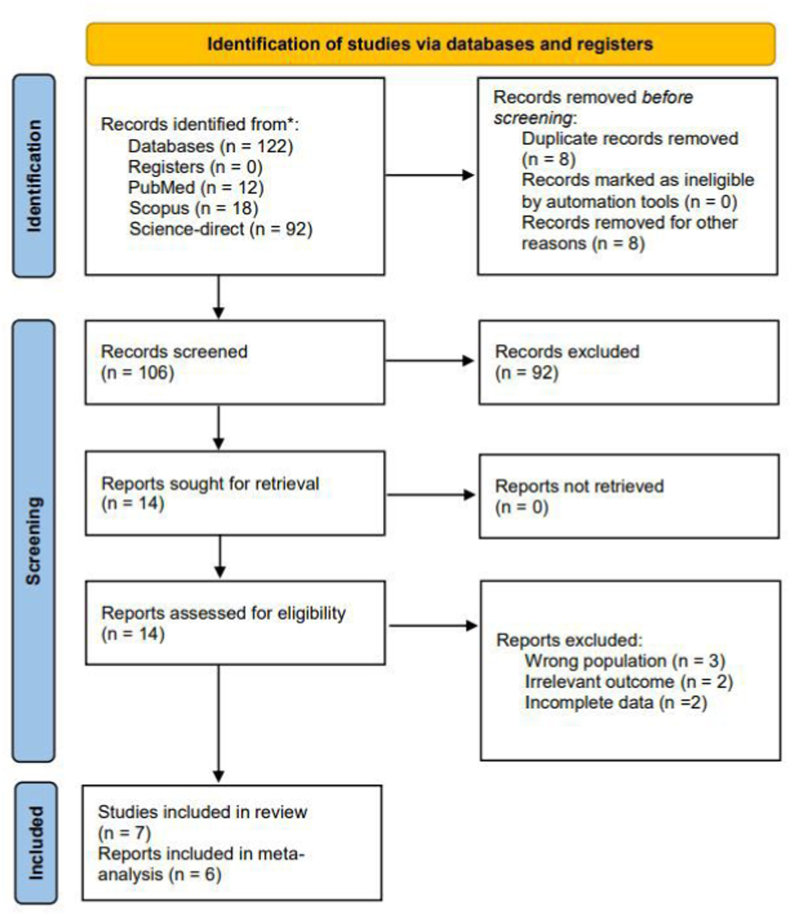
PRISMA Flow diagram [13] (Fig. 1) demonstrates the article searching and selection process. Our initial search from multiple databases yielded a total of 122 records. Sixteen articles were removed due to duplicates and other reasons (non-original article and animal study), leaving 106 articles to be screened through the Mendeley reference manager. After the primary screening process, fourteen studies were further evaluated in full text. The final analysis included seven articles [7,[9], [10], [11], [12],16,17] for the systematic review, six of which [7,[9], [10], [11], [12],16] were eligible to be included in the meta-analysis.
Fig. 1.
Systematic Search and Screening based on the 2020 PRISMA flow diagram.
3.2. Study characteristics
All the included studies were single-institutional retrospective cohorts, except for a multi-institutional retrospective cohort by Li et al. [16]. Studies were mostly conducted in the Asian population, except for Azizi et al. [10], which was conducted in the American population. Participants from the included studies had an average age ranging from 50.6 years to 68.2 years. The median study follow-up ranged from 18 months to 35.5 months, as summarized in Table 2. All the participants in the included studies had undergone inguinal lymph node dissection (ILND), with pathological confirmation of penile squamous cell carcinoma (SCC). Table 3 summarizes the outcome assessment in the included studies, while Table 4 summarizes the results of the oncological outcome for penile SCC with high NLR compared to low NLR. Studies used different cut-off values to determine high and low NLR, ranging from 2.6 to 3.59. There were various methods for determining optimal NLR cut-off values, including the area under the curve (AUC), receiver operating characteristic (ROC), and Contal and O'Quigley methods. The median OS was not described in many of the included studies, and only Azizi et al. [10] and Chen Hu et al. [12] reported the median patients' OS of 89 months and 34 months, respectively.
Table 2.
Baseline characteristics of the included study.
| No | Author & years of publication | Years of Data Collection | Patient's TNM Staging | Country | Study Design | Total patients (n) | Group Allocation | Allocated patients (n) | Cancer Pathology | Age in years (mean ± SD) | Follow-up duration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kasuga et al., 2016 | 1999–2015 | T1, T2, T3, T4 N0, N + M0, M+ | Japan | Single Institutional Retrospective Cohort | 41 | High NLR | 20 | Penile SCC | 68.5 ± 11.4 | 34.7 (2.3–271.7) monthsb |
| Low NLR | 21 | ||||||||||
| 2 | Tan et al., 2017 | 2007–2015 | Ta, T1a, T1b, T2, T3 | Singapore | Single Institutional Retrospective Cohort | 39 | High NLR | NR | Penile SCC | 65. (59–72.5)b | 34 (16.5–66) monthsb |
| N0, N1, N2, N3 | Low NLR | NR | |||||||||
| 3 | Azizi et al., 2018 | 1994–2014 | Tx, Tis/Ta/T1a, T1b, T2, T3, T4 | USA | Single Institutional Retrospective Cohort | 84 | High NLR | 38 | Penile SCC | 63.6(54–68.7)a 68.2(53.4–73.6)a | 35.5 (19.4–89.6) monthsb |
| N0, N+ | Low NLR | 30 | |||||||||
| 4 | Li et al., 2019 | 2002–2015 | ≤T1, ≥T2 | China | Multi- Institutional Retrospective cohort | 228 | High NLR | 105 | Penile SCC | 52(24–85)a | 25 (1–140) monthsa |
| N0, N+ | Low NLR | 123 | |||||||||
| 5 | Jiao Hu et al., 2020 | 2010–2018 | Tis, Ta/T1a, ≥T1b | China | Single Institutional Retrospective Cohort | 134 | High NLR | 32 | Penile SCC | 54.95 ± 10.6 | 32.1(2–94) montha |
| No, N+ | Low NLR | 47 | |||||||||
| 6 | Chen Hu et al., 2020 | 2002–2017 | T0, T1, T2, T3, T4 | China | Single Institutional Retrospective Cohort | 225 | High NLR | 68 | Penile SCC | 50.6 ± 13.4 | 30 (16–63.5) monthsb |
| N0, N1, N2, N3 M0, M1 | Low NLR | 157 | |||||||||
| 7 | Jindal et al., 2021 | 2012–2020 | T1, T2, T3, T4 | India | Single Institutional Retrospective Cohort | 69 | High NLR | 40 | Penile SCC | NR | 18 (2–74) monthsa |
| N0, N1, N2, N3 | Low NLR | 29 |
Data expressed as median and range.
Data expressed as median and interquartile (IQR) range, SCC = Squamous cell carcinoma.
Table 3.
Outcome assessment and treatment received in the included studies.
| No | Author & years of publication | Surgical Intervention | NLR (mean ± SD) | NLR Cut-off | Cut-off point calculation | Median OS | Median CSS | Primary Outcome | Secondary Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Kasuga et al., 2016 | Radical Penectomy | 5.03 ± 4.99 | 2.82 | AUC | NR | NR | OS, CSS | LNM |
| 2 | Tan et al., 2017 | Bilateral modified inguinal lymph node dissection or dynamic sentinel node biopsy | 2.99 (0.76–5.22)∗∗ | 2.8 | AUC | NR | NR | CSS, PFS, | LNM |
| 3 | Azizi et al., 2018 | Bilateral/unilateral inguinal lymph node dissection | 4.51 ± 3.95 | 3 | Contal and O'Quigley method | 89 (31.2–123.6) months∗∗ | NR | OS, CSS, RFS | LNM |
| 4 | Li et al., 2019 | Bilateral inguinal lymph node dissection | 2.4 (0.1–44.2)∗ | 2.6 | ROC | NR | NR | CSS | LNM |
| 5 | Jiao Hu et al., 2020 | Bilateral inguinal lymph node dissection after primary tumor procedure | NR | 3.59 | ROC | NR | NR | CSS | LNM |
| 6 | Chen Hu et al., 2020 | Inguinal lymph node dissection | NR | 2.94 | AUC | 34 (18–84) months∗∗ | 33 (18–85.25) months∗∗ | OS, PFS | LNM |
| 7 | Jindal et al., 2021 | Penectomy with bilateral inguinal node dissection | NR | 3 | Following previous study by Azizi et al. | NR | 18 (2–74) months∗ | CSS | LNM |
NLR = Neutrophil-lymphocyte ratio, ROC = Receiver operating characteristic curve, AUC = Area under the curve, OS = Overall Survival, CSS = Cancer specific-survival, PFS = Progression-free survival, LNM = Lymph-Node Metastasis.
Data expressed as median and range.
Data expressed as median and interquartile (IQR) range.
Table 4.
Oncological outcomes results summary for penile SCC with high NLR compared to low NLR.
| Outcome | Analysis methods | Reference | Result | p value |
|---|---|---|---|---|
| Overall Survival | Univariate Analysis | Kasuga et al., 2016 | NR | 0.076 |
| Chen Hu et al., 2020 | HR: 2.97 (95% CI 1.74 to 5.06) | <0.001 | ||
| Multivariate Analysis | Chen Hu et al., 2020 | HR: 1.28 (95% CI 0.61 to 2.72) | 0.516 | |
| Azizi et al., 2018 | HR: 2.48 (95% CI 1.02 to 6.03) | 0.046 | ||
| Cancer-Specific Survival | Univariate Analysis | Kasuga et al., 2016 | NR | 0.023 |
| Azizi et al., 2018 | HR: 6.16 (95% CI 2.1 to 18.07) | 0.014 | ||
| Jiao Hu et al., 2020 | HR: 3.36 (95% CI 1.49 to 7.58) | <0.01 | ||
| Jindal et al., 2021 | NR | 0.05 | ||
| Multivariate Analysis | Tan et al., 2017 | NR | <0.01 | |
| Azizi et al., 2018 | HR: 2.58 (95% CI 0.79 to 8.43) | 0.116 | ||
| Li et al., 2019 | HR: 2.25 (95% CI 1.12 to 4.51) | 0.023 | ||
| Jiao Hu et al., 2020 | HR: 1.27 (95% CI 0.28 to 5.70) | 0.76 | ||
| Jindal et al., 2021 | NR | 0.94 | ||
| Lymph-Node Metastasis | Univariate Analysis | Azizi et al., 2018 | OR: 3.75 (95% CI 1.30 to 10.81) | 0.014 |
| Chen Hu et al., 2020 | OR: 2.21 (95% CI 1.23 to 3.99) | 0.008 | ||
| Jiao Hu et al., 2020 | OR: 6.92 (95% CI 2.46 to 19.43) | <0.01 | ||
| Jindal et al., 2021 | OR: 6.13 (95% CI 2.13 to 17.65) | 0.001 | ||
| Kasuga et al., 2016 | OR: 5.12 (95% CI 0.91 to 28.64) | 0.049 | ||
| Multivariate Analysis | Azizi et al., 2018 | OR: 3.66 (95% CI 0.82 to 16.34) | 0.091 | |
| Jiao Hu et al., 2020 | OR: 10.93 (95% CI 2.81 to 42.51) | <0.01 | ||
| Jindal et al., 2021 | NR | 0.09 |
OR = Odds ratio, HR = Hazard Ratio, NR = Not reported.
Additionally, Chen Hu et al. [12] found that patients with high NLR had a shorter OS than patients with low NLR, with a median of 30 months and 158 months, respectively. In addition, the median CSS was also not described in the results of most of the studies. However, Chen Hu et al. [12] and Jindal et al. [7] reported that the penile cancer patients in their cohort had a median CSS of 33 months and 18 months, respectively. The value of NLR was obtained from a complete blood count examination taken from peripheral blood samples prior to the surgical procedure which the course varied from 1 month to 3 days before inguinal lymph node dissection surgery.
3.3. Risk of bias
All the included studies in the meta-analysis were retrospective cohorts, and the quality of these studies was assessed using the NOS [15], which was designed explicitly for observational studies. This scale evaluates each article's selection, comparability, and outcome domain. Since available data from medical records and control populations were selected from the same population as the exposed population, all studies were scored with a good score in the NOS selection domain. Most studies had controlled for various factors that could affect the outcomes such as age, based on multivariate analysis and fulfilled a good comparability score on NOS. Similarly, most of the included articles in this review had reported adequate follow-up duration and description, so the NOS outcome domain was also good. In general, the overall NOS evaluation revealed that all the included studies had a good quality, as presented in Table 5.
Table 5.
Risk of bias assessment using the Newcastle-Ottawa Scale.
| Authors | Selection | Comparability | Outcome | Total Score |
|---|---|---|---|---|
| Tindal et al., 2021 | *** | ** | ** | 7 |
| Hu Jiao et al., 2020 | *** | ** | *** | 8 |
| Zaishang et al., 2020 | **** | ** | *** | 9 |
| Hu Chen et al., 2020 | *** | ** | *** | 8 |
| Azizi et al., 2018 | **** | ** | *** | 9 |
| Kasuga et al., 2016 | *** | ** | *** | 8 |
3.4. Meta-analysis results on the lymph node invasion
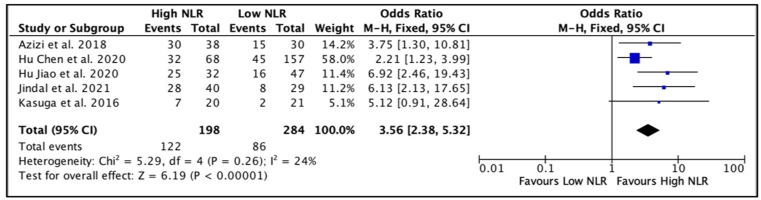
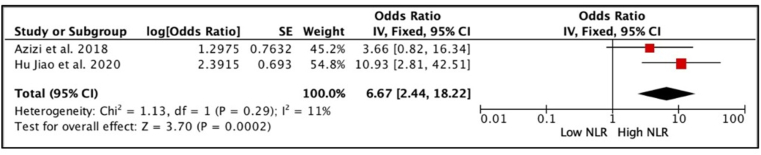
The meta-analysis included five studies [7,[9], [10], [11], [12]], totalling 482 patients with penile cancer who underwent inguinal node dissection. Based on the forest plot analysis, patients with high NLR values had a higher incidence of lymph node invasion compared to patients with low NLR (OR 3.56, 95% CI 2.38, 5.32, p < 0.01) (Fig. 2). Furthermore, a meta-analysis performed on the multivariate analysis by Azizi et al. [10] and Hu Jiao et al. [9] (Fig. 3) showed that penile cancer with high NLR had a significantly worse lymph node invasion than patients with low NLR (OR 6.67, 95% CI 2.44, 18.22, p < 0.01). The heterogeneity between studies was non-significant, with an I2 value of 24% and 11% for univariate analysis and multivariate analysis, respectively. Therefore, the analysis model used for this outcome was fixed effects.
Fig. 2.
Forest plot comparison of unadjusted OR of node invasion in penile cancer patients with high NLR versus low NLR.
Fig. 3.
Forest plot comparison of adjusted OR of lymph node invasion in penile cancer patients with high NLR versus low NLR.
3.5. Meta-analysis results on the cancer-specific survival
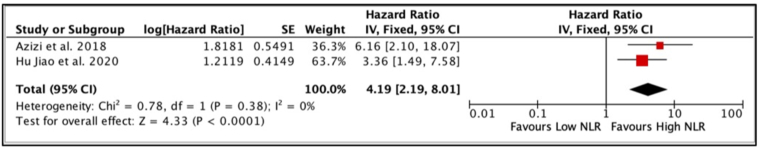
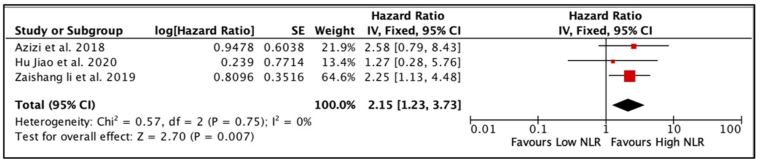
Three articles [9,10,16] with a total of 446 patients with penile carcinoma were analysed for CSS. According to the combined meta-analysis of univariate analysis, penile carcinoma patients with high NLR scores had significantly worse CSS than those with low NLR, with a HR of 4.19 (95% CI 2.19, 8.01, p = 0.01) (Fig. 4). Furthermore, the combined analysis of the three studies' multivariate analysis also revealed similar findings (HR 2.15, 95% CI 1.23, 3.73, p < 0.01) (Fig. 5). The chi-square and I2 tests used to analyse heterogeneity between studies revealed low heterogeneity (I2 = 0%). Thus, the fixed-effects model was selected.
Fig. 4.
Forest plot comparison of unadjusted HR of CSS in penile cancer patients with high NLR versus low NLR.
Fig. 5.
Forest plot comparison of adjusted HR of CSS in penile cancer patients with high NLR versus low NLR.
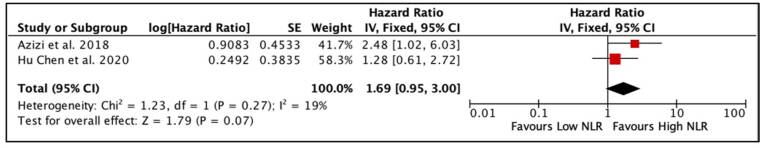
3.6. Meta-analysis results on the overall survival
Meta-analysis on the OS comprised two studies [10,12] with 309 penile cancer patients. Based on the forest plot results shown in Fig. 6, penile cancer patients with high NLR had a similar OS to those with low NLR (HR 1.69, 95% CI 0.95, 3, p = 0.07). The I2 index analysis on the forest plot shows no significant heterogeneity between the included studies (I2 = 19%), implying that the analysis model used for meta-analysis was fixed-model effects.
Fig. 6.
Forest plot comparison of adjusted HR of OS in penile cancer patients with high NLR versus low NLR.
4. Discussion
The presence of lymph node involvement signifies locoregional cancer metastasis. Although the presence of metastatic disease in the lymph nodes has been found as an independent predictor of survival in penile cancer, lymphadenectomy for penile cancer does not always procure positive cancer cells and can be associated with false-negative results. Considering all these findings, the beneficial and harmful effects of lymph node examination should be cautiously interpreted. Studies have reported that PD-L1, squamous cell antigen, C-reactive protein, and P53 are all possible predictors for LNM in penile cancer [12]. Platelet to Lymphocyte Ratio (PLR) is another putative biomarker that can be detected by a simple complete blood count (CBC) examination [12]. NLR, on the other hand, has additional advantages as a useful predictor because it is affordable, repeatable, and can be utilized in remote locations.
NLR was found to be associated with LNM in this study, and it was also found to be an independent predictor for lymph node involvement. Forest plot analyses revealed that high NLR was associated with an increased probability of LNM. The Forest plot of the adjusted OR also showed that high NLR was an independent predictor for LNM. However, given that only two studies were included in this analysis, the conclusion should be taken cautiously.
Cancer patient with LNM has a poorer prognosis than those without the nodal disease. Tumor cells released substances that interact with stromal, myeloid, and lymphoid cells in primary tumors and the lymphatic system, lowering antitumor immunity and encouraging tumor growth, thus increasing the metastatic process [18]. The early detection of nodal invasion from the primary tumor is a critical point to adjust sufficient management. Previous studies have shown that NLR is a potential predictor for lymph node involvement during cancer progression. Higher pre-treatment NLR was identified as a predictor of LNM in endometrial cancers [19]. A study by Zhou et al. highlighted the role of NLR in association with LNM that is determinate on multivariable analysis (NLR ≥1.80) for patients with pancreatic neuroendocrine tumors and similarly in pancreatic ductal adenocarcinoma [20]. Xu et al. investigated that preoperative peripheral blood NLR concentration confers a higher risk of LNM in medullary thyroid carcinoma patients [21]. Other evidence of NLR as a useful complementary diagnostic tool for predicting pathological node involvement is observed in gastric cancer, notably because of its higher sensitivity and negative predictive value [22,23]. While most studies support the theory that NLR is associated with LNM, several studies demonstrated contradictive results. A study in 2016 by Maeda et al. discussed the lack of an association between NLR and PSA failure in prostate cancer patients who underwent Radical Prostatectomy. At the end of their study, they found no significant correlations of NLR with LNM (p = 0.062) [24]. A study by Yersal et al. (2017) about NLR and PLR in breast cancer subtypes also stated that there were no significant correlations between NLR or PLR and LNM count (p = 0.276). The different results of all this evidence should be cautiously interpreted, and the tumor burden might result in different tumor micro-environment and immune responses that affect NLR [25].
High NLR is also associated with worse outcomes in some malignancies, including urology malignancies. A meta-analysis by Wei et al. demonstrated that NLR was a predictor for worse CSS in bladder cancer, prostate cancer, and renal cell carcinoma [26]. Similar result by Shao et al. in renal cell carcinoma [27]. The Forest plot of this study showed that high NLR was an independent predictor for worse CSS in penile cancer. Nevertheless, our study shows no significant difference in OS based on NLR stratification. The latest study suggested that increasing pre-treatment neutrophil-to-lymphocyte ratio is related to the more severe clinical and pathological outcome as an independent predictor, including in testicular cancer. Staging of the tumors, the tendency to recurrence, and CSS are worse with higher NLR [28]. Higher post-orchiectomy NLR was found independently associated with recurrence in testicular cancer patients [29]. Among advanced/metastatic bladder cancer patients, NLR could independently improve the prediction of survival outcomes. Higher NLR is strongly linked to a poorer OS, with a HR of 5.06 in one study and the CSS of 36% lower in another study [30,31]. The patients with an NLR of ≤3.0 are likely to have better cancer outcomes and attain greater survival improvement from chemotherapy compared to the group with NLR of >3.0.
Several malignancies in different sites of system organs have been associated with poor clinical outcomes in the presence of increased NLR. A meta-analysis evaluating patients with breast cancer found a worse OS amongst greater NLR than the cut-off value [32]. An increased NLR indicated poor outcomes in patients with hepatocellular carcinoma, reflected in both OS and recurrence-free survival (RFS), according to one meta-analysis [33]. This finding has been confirmed in other prognostic studies on gastrointestinal cancer, head and neck cancer, and gynaecological cancer [[34], [35], [36], [37]]. Quantitative studies presented suggest an association between elevated NLR and poor clinical and survival outcomes across a wide spectrum of diagnoses, stages of the disease, and course of treatment.
However, several studies have found a different outcome pattern for NLR. Ojerholm et al. conducted a study about NLR as a bladder cancer biomarker, assessing prognostic and predictive values in SWOG 8710, and reported that NLR was neither a prognostic (p = 0.24) nor predictive (p = 0.86) biomarker for OS in muscle-invasive bladder cancer [38]. In contrast, Marchioni et al. demonstrated that a high NLR was associated with poorer oncological outcomes in patients affected by UTUC in terms of OS and RFS but not in cancer-specific survival (p = 0.77) based on a systematic review and meta-analysis study on high NLR as a prognostic factor in patients affected by Upper Tract Urothelial Cancer (UTUC) [39]. The different results of NLR as a prognostic factor in survival might be correlated to the indiscriminate role in tissue inflammation, the dichotomy of value measurement, and that most studies reporting NLR were observational in nature that were exposed to potential biases including the heterogeneous dataset.
The NLR has potential role to predict lymph node involvement in penile cancer. However, the difference in the NLR cut-off value was observed in this study. On the one hand, NLR indicators that are dichotomously distinct can have strong predictive significance for LNM and CSS, while in the other hand, the variations in cut-off values among published studies indicates that there are several variables which may influence the determination of NLR cut-off value. The determination of NLR mostly came from AUROC (Area Under Receiver Operating Characteristic) curve analysis. Each study assigned a different cut-off value based on ROC curve result which was influenced by study own's population. Since NLR value was derived from complete blood count examination, the patient's laboratory parameter and patient's demographic characteristic play significant role in affecting the result. The influence of race and age of the patients are important attributes in determination of NLR value. Furthermore, comorbidities and the degree of systemic inflammation in study's population may also contribute to the value.
Our systematic review and meta-analysis are not without limitations. First, all the included studies were retrospective in nature and will be susceptible to certain biases from a lack of standardized inclusion criteria, treatment schemes, and follow-up schedules. Second, there was no established (or standardised) cut-off value of NLR. Each study assigned a cut-off value with various methods based on the highest sensitivity and specificity from AUROC curve or used predefined cut-off values derived from other studies. Third, the population of this study are mostly Asian patients which does not represent the world population. Nonetheless, the inclusion of all published studies and critical analysis of the effect of NLR while controlling for other variables in LNM, CSS, and OS are strengths of this study. It is likely that large-scale prospective studies with a well-designed methodology are needed to establish the role of NLR in penile cancer.
5. Conclusion
This study demonstrated the value of NLR as an independent predictor for LNM and CSS in penile cancer. However, NLR in not proven to be an independent predictor for OS.
Ethics committee approval
This systematic review and meta-analysis do not require an ethical approval.
Funding source
None.
Author contribution
-
•
Haviv Muris Saputra (H.M.S.) is involved in the concept and project design, materials, literature search, data collection and/or processing, analysis and/or interpretation, writing the manuscript, and final approval of the version to be submitted.
-
•
Furqan Hidayatullah (F.H.) is involved in the materials, literature search, data collection and/or processing, analysis and/or interpretation, writing the manuscript, and final approval of the version to be submitted.
-
•
Yudhistira Pradnyan Kloping (Y.P.K.) is involved in the materials, literature search, data collection and/or processing, analysis and/or interpretation, writing the manuscript, and final approval of the version to be submitted.
-
•
Johan Renaldo (J.R.) is involved in the concept and project design, supervision, resources, materials, literature search, data collection and/or processing, analysis and/or interpretation, writing the manuscript, and final approval of the version to be submitted.
-
•
Eric Chung (E.C.) is involved in the supervision, resources, materials, writing the manuscript, and final approval of the version to be submitted.
-
•
Lukman Hakim (L.H.) is involved in the concept and project design, supervision, resources, materials, literature search, data collection and/or processing, analysis and/or interpretation, writing the manuscript, and final approval of the version to be submitted.
Guarantor
Lukman Hakim, Department of Urology, Faculty of Medicine, Universitas Airlangga, Universitas Airlangga Teaching Hospital, Indonesia. Email: lukman-h@fk.unair.ac.id.
Informed consent
This systematic review and meta-analysis do not need an informed consent statement.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgement
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.104335.
Contributor Information
Haviv Muris Saputra, Email: haviv.saputra@gmail.com.
Furqan Hidayatullah, Email: furqanhidayatullah26@gmail.com.
Yudhistira Pradnyan Kloping, Email: yudhistira.pradnyan.kloping-2020@fk.unair.ac.id.
Johan Renaldo, Email: joeurologi@gmail.com.
Lukman Hakim, Email: lukman-h@fk.unair.ac.id.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Morris B.J., Gray R.H., Castellsague X., Bosch F.X., Halperin D.T., Waskett J.H., Hankins C.A. The strong protective effect of circumcision against cancer of the penis. Adv. Urol. 2011;2011:1–21. doi: 10.1155/2011/812368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heller D. Lesions and neoplasms of the penis: a review. J. Lower Genital Tract Dis. 2016;20(1):107–111. doi: 10.1097/LGT.0000000000000159. https://scholarship.libraries.rutgers.edu/discovery/delivery/01RUT_INST:ResearchRepository/12643417590004646?l#13643521460004646Heller Internet. Available from: https://doi.org/10.7282/T3P84DXNPublishedVersion:http://dx.doi.org/10.1097/LGT.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 3.Coelho R.W.P., Pinho J.D., Moreno J.S., Garbis D.V.E.O., do Nascimento A.M.T., Larges J.S., Calixto J.R.R., Ramalho L.N.Z., da Silva A.A.M., Nogueira L.R., de Moura Feitoza L., Silva G.E.B. Penile cancer in Maranhão, Northeast Brazil: the highest incidence globally? BMC Urol. 2018;18(1) doi: 10.1186/s12894-018-0365-0. May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakenberg O.W., Compérat E.M., Minhas S., Necchi A., Protzel C., Watkins N. 2021. EAU Guidelines on Penile Cancer. [DOI] [PubMed] [Google Scholar]
- 5.Culine S., Kramar A., Théodore C., Geoffrois L., Chevreau C., Biron P., Nguyen B.B., Héron J.F., Kerbrat P., Caty A., Delva R., Fargeot P., Fizazi K., Bouzy J., Droz J.P. Randomized trial comparing bleomycin/etoposide/cisplatin with alternating cisplatin/cyclophosphamide/doxorubicin and vinblastine/bleomycin regimens of chemotherapy for patients with intermediate- and poor-risk metastatic nonseminomatous germ cell tumors: genito-Urinary Group of the French Federation of Cancer Centers Trial T93MP. J. Clin. Oncol. 2008 Jan 20;26(3):421–427. doi: 10.1200/JCO.2007.13.8461. [DOI] [PubMed] [Google Scholar]
- 6.Hakenberg O.W., Protzel C. Chemotherapy in penile cancer. Therap. Adv. Urol. 2012;4(3):133–138. doi: 10.1177/1756287212441235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jindal T., Pawar P., Agarwal S., Jain P., Meena M., Sarwal A., Dhanalakshmi M. The use of preoperative neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio in predicting survival and groin node involvement of patients with squamous cell carcinoma of penis. Urol. Ann. 2021 Oct 1;13(4):391–396. doi: 10.4103/UA.UA_112_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Çalışkan S., Kaba S., Özsoy E., Koca O., Akyüz M. Öztürk Mİ. The hematological parameters in testicular cancer. J. Oncol. Sci. 2017;3(3):117–119. [Google Scholar]
- 9.Hu J., Li H., He T., Deng H., Gong G., Cui Y., Liu P., Ren W., Li C., Chen J., Zu X. A nomogram incorporating PD-L1, NLR, and clinicopathologic features to predict inguinal lymph node metastasis in penile squamous cell carcinoma. Urologic Oncology. Sem. Orig. Investig. 2020;38(7) doi: 10.1016/j.urolonc.2020.04.015. Jul 1, 641.e19-641.e29. [DOI] [PubMed] [Google Scholar]
- 10.Azizi M., Peyton C.C., Boulware D.C., Chipollini J., Juwono T., Pow-Sang J.M., Spiess P.E. Prognostic value of neutrophil-to-lymphocyte ratio in penile squamous cell carcinoma patients undergoing inguinal lymph node dissection. European Urology Focus. 2019;5(6):1085–1090. doi: 10.1016/j.euf.2018.06.008. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasuga J., Kawahara T., Takamoto D., Fukui S., Tokita T., Tadenuma T., Narahara M., Fusayasu S., Terao H., Izumi K., Ito H., Hattori Y., Teranishi J.I., Sasaki T., Makiyama K., Miyoshi Y., Yao M., Yumura Y., Miyamoto H., Uemura H. Increased neutrophil-to-lymphocyte ratio is associated with disease-specific mortality in patients with penile cancer. BMC Cancer. 2016;16(1) doi: 10.1186/s12885-016-2443-6. Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu C., Bai Y., Li J., Zhang G., Yang L., Bi C., Zhao B., Yang Y., Li R., Wu H., Wang Q., Qin Y. Prognostic value of systemic inflammatory factors NLR, LMR, PLR and LDH in penile cancer. BMC Urol. 2020;20(1) doi: 10.1186/s12894-020-00628-z. May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 14.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., Henry D.A. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358 doi: 10.1136/bmj.j4008. http://www.bmj.com/content/358/bmj.j4008.abstract Internet. Sep. 21, : j4008. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells G.A., Connell D.O., Peterson J., Welch V., Losos M., P Tugwell B.S. Newcastle-ottawa quality assessment scale. Ottawa hospital research institute. OHRI. 2014;3:2–4. [Google Scholar]
- 16.Li Z., Li X., Zhang X., Chen P., Wang B., Chen X., Han H., Zhou F. Prognostic significance of common preoperative laboratory variables in penile squamous cell carcinoma. Int. J. Urol. 2020;27(1):76–82. doi: 10.1111/iju.14137. [DOI] [PubMed] [Google Scholar]
- 17.Tan T.W., Chia S.J., Chong K.T. Management of penile cancer in a Singapore tertiary hospital. Arab J Urol. 2017;15(2):123–130. doi: 10.1016/j.aju.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones D., Pereira E.R., Padera T.P. Growth and immune evasion of lymph node metastasis. Front. Oncol. 2018;8(FEB) doi: 10.3389/fonc.2018.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoyama T., Takano M., Miyamoto M., Yoshikawa T., Kato K., Sakamoto T., Takasaki K., Matsuura H., Soyama H., Hirata J., Suzuki A., Sasa H., Tsuda H., Furuya K. Pretreatment neutrophil-to-lymphocyte ratio was a predictor of lymph node metastasis in endometrial cancer patients. Oncology. 2019;96(5):259–267. doi: 10.1159/000497184. [DOI] [PubMed] [Google Scholar]
- 20.Zhou B., Deng J., Chen L., Zheng S. Preoperative neutrophil-to-lymphocyte ratio and tumor-related factors to predict lymph node metastasis in nonfunctioning pancreatic neuroendocrine tumors. Sci. Rep. 2017;7(1):1–10. doi: 10.1038/s41598-017-17885-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang K., Lei J., Chen W., Gong Y., Luo H., Li Z., Gong R., Zhu J. Association of the preoperative neutrophil-tolymphocyte and platelet-to-lymphocyte ratios with lymph node metastasis and recurrence in patients with medullary thyroid carcinoma. Medicine. 2016;95(40):1–6. doi: 10.1097/MD.0000000000005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng D., Lin Y., Yu Y., Wang K., Ouyang X., Dang Y., Huang Q. The value of preoperative neutrophil to lymphocyte ratio in indicating lymph node metastasis in patients with resectable T2 stage gastric adenocarcinoma. Clin. Lab. 2016;62(4):659–665. doi: 10.7754/clin.lab.2015.150824. [DOI] [PubMed] [Google Scholar]
- 23.Kosuga T., Konishi T., Kubota T., Shoda K., Konishi H., Shiozaki A., Okamoto K., Fujiwara H., Kudou M., Arita T., Morimura R., Murayama Y., Kuriu Y., Ikoma H., Nakanishi M., Otsuji E. Clinical significance of neutrophil-to-lymphocyte ratio as a predictor of lymph node metastasis in gastric cancer. BMC Cancer. 2019;19(1):1–7. doi: 10.1186/s12885-019-6404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda Y., Kawahara T., Koizumi M., Ito H., Kumano Y., Ohtaka M., Kondo T., Mochizuki T., Hattori Y., Teranishi J.I., Yumura Y., Miyoshi Y., Yao M., Miyamoto H., Uemura H. Lack of an association between neutrophil-to-lymphocyte ratio and PSA failure of prostate cancer patients who underwent radical prostatectomy. BioMed Res. Int. 2016:2016. doi: 10.1155/2016/6197353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yersal Ö Çetinkünar S., Aktimur R., Aziret M., Özdas S., Erdem H., Yildirim K. Neutrophil/lymphocyte and platelet/lymphocyte ratios are not different among breast cancer subtypes. Asian Pac. J. Cancer Prev. APJCP. 2017;18(8):2227–2231. doi: 10.22034/APJCP.2017.18.8.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Y., Jiang Y.Z., Qian W.H. Prognostic role of NLR in urinary cancers: a meta-analysis. PLoS One. 2014;9(3):1–6. doi: 10.1371/journal.pone.0092079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao Y., Wu B., Jia W., Zhang Z., Chen Q., Wang D. Prognostic value of pretreatment neutrophil-to-lymphocyte ratio in renal cell carcinoma: a systematic review and meta-analysis. BMC Urol. 2020;20(1) doi: 10.1186/s12894-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan Y.G., Sia J., Huang H.H., Lau W.K.O. Neutrophil-to-lymphocyte ratio independently predicts advanced pathological staging and poorer survival outcomes in testicular cancer. Investig. Clin. Urol. 2019;60(3):176–183. doi: 10.4111/icu.2019.60.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauzá Quetglas J.L., Tienza Fernández A., Bertolo R., Sabaté Arroyo X.A., Guimerà García J., Tubau Vidaña V., Frontera Juan G., Pieras Ayala E. The prognostic value of the neutrophil-to-lymphocyte ratio in patients with testicular cancer. Progres en urologie. Journal De l’Association Francaise D’urologie Et De La Societe Francaise D’urologie. 2020 Apr;30(5):273–280. doi: 10.1016/j.purol.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Tan Y.G., Eu E.W.C., Huang H.H., Lau W.K.O. High neutrophil-to-lymphocyte ratio predicts worse overall survival in patients with advanced/metastatic urothelial bladder cancer. International journal of urology. Off. J. Jpn. Urol. Assoc. 2018 Mar;25(3):232–238. doi: 10.1111/iju.13480. [DOI] [PubMed] [Google Scholar]
- 31.Tan Y.G., Eu E., Lau Kam On W., Huang H.H. Pretreatment neutrophil-to-lymphocyte ratio predicts worse survival outcomes and advanced tumor staging in patients undergoing radical cystectomy for bladder cancer. Asian J Urol. 2017 Oct;4(4):239–246. doi: 10.1016/j.ajur.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ethier J.L., Desautels D., Templeton A., Shah P.S., Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):1–13. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng J., Cai J., Li H., Zeng K., He L., Fu H., Zhang J., Chen L., Yao J., Zhang Y., Yang Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry. Pharmacology. 2017;44(3):967–981. doi: 10.1159/000485396. [DOI] [PubMed] [Google Scholar]
- 34.Haram A., Boland M.R., Kelly M.E., Bolger J.C., Waldron R.M., Kerin M.J. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J. Surg. Oncol. 2017 Mar;115(4):470–479. doi: 10.1002/jso.24523. [DOI] [PubMed] [Google Scholar]
- 35.Mascarella M.A., Mannard E., Silva S.D., Zeitouni A. Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: a systematic review and meta-analysis. Head Neck. 2018 May;40(5):1091–1100. doi: 10.1002/hed.25075. [DOI] [PubMed] [Google Scholar]
- 36.Huang Q.T., Zhou L., Zeng W.J., Ma Q.Q., Wang W., Zhong M., Yu Y.H. Prognostic significance of neutrophil-to-lymphocyte ratio in ovarian cancer: a systematic review and meta-analysis of observational studies. Cell. Physiol. Biochem. 2017;41(6):2411–2418. doi: 10.1159/000475911. [DOI] [PubMed] [Google Scholar]
- 37.Ni L., Tao J., Xu J., Yuan X., Long Y., Yu N., Wu R., Zhang Y. Prognostic values of pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in endometrial cancer: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020;301(1):251–261. doi: 10.1007/s00404-019-05372-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojerholm E., Smith A., Hwang W ting, Baumann B.C., Tucker K.N., Lerner S.P., Mamtani R., Boursi B., Christodouleas J.P. vol. 123. 2018. pp. 794–801. (Assessing Prognostic and Predictive Value in SWOG 8710). 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchioni M., Cindolo L., Autorino R., Primiceri G., Arcaniolo D., De Sio M., Schips L. High neutrophil-to-lymphocyte ratio as prognostic factor in patients affected by upper Tract urothelial cancer: a systematic review and meta-analysis. Clin. Genitourin. Cancer. 2017;15(3):343–349. doi: 10.1016/j.clgc.2016.12.027. e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.