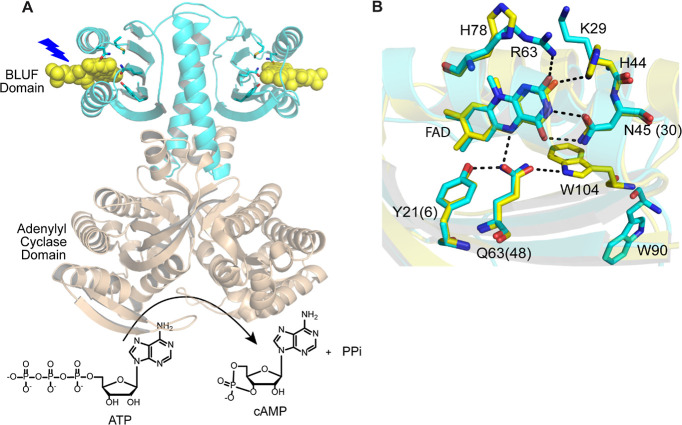
Figure 1.
Structure of OaPAC and the flavin binding pocket. (A) OaPAC is a homodimer composed of a BLUF domain (cyan color) linked to an AC domain (sand color) responsible for converting ATP into cAMP and PPi in a light-dependent manner (PDB: 5x4t).18 (B) Hydrogen-bonding network that surrounds the isoalloxazine ring of the FAD. The structure of OaPAC (cyan) has been superimposed on the structure of AppA (yellow; PDB: 1YRX) where the tryptophan (W104) is in the Trpin conformation.12 Residue numbers are for AppA, while those in parentheses are for OaPAC.