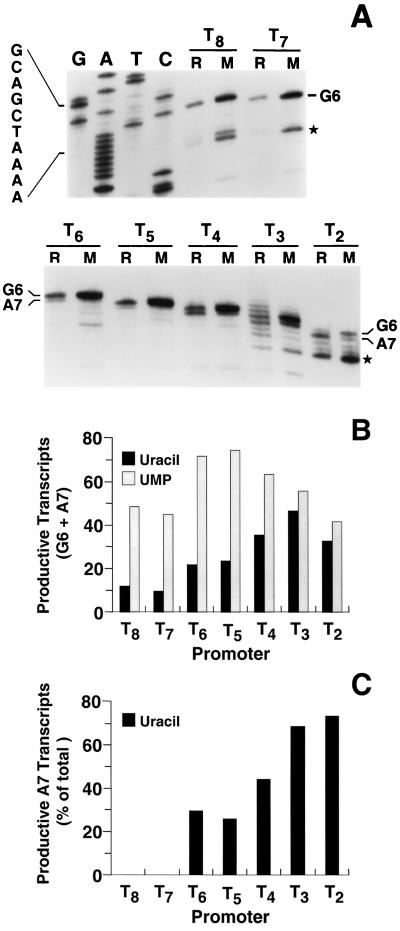
FIG. 2.
Levels of upp::lacZ transcripts initiated at the wild-type (T8) and mutant (Tn) promoters. Cellular RNA was quantitatively isolated from cells grown on either uracil (R) or UMP (M). Transcript levels were measured by primer extension mapping as described in Materials and Methods. (A) Autoradiograph of the 10% polyacrylamide sequencing gel that was used to separate the primer extension products. The autoradiograph was cut between the T7 and T6 lanes, and one part was placed beneath the other to facilitate labeling. The dideoxy sequencing ladder of the wild-type upp promoter region, which was used to identify transcripts, was generated with the same primer that was used for primer extension. (Note that the DNA sequence is for the template strand and is complementary to the transcript sequences.) The bands corresponding to G6 and (where detected) A7 transcripts are indicated. The positions of these bands in the gel are different for each promoter, reflecting the number of U residues specified by the T · A tract. Bands marked with an asterisk represent major transcripts produced by in vivo degradation of G6 and A7 transcripts. (B) Total productive (i.e., upp::lacZ) transcript levels in uracil- and UMP-grown cells were quantitated using a PhosphorImager and plotted in arbitrary units. Total transcript levels included G6 and A7 transcripts, degradation products, and escaped stuttering products in the case of the T3 fusion. (C) The levels of productive A7-initiated transcripts in uracil-grown cells were plotted as percentages of total productive transcript levels, calculated using the formula (A7/G6 + A7) × 100. In the case of the T3 fusion, escaped stuttering products were counted as A7 transcripts.