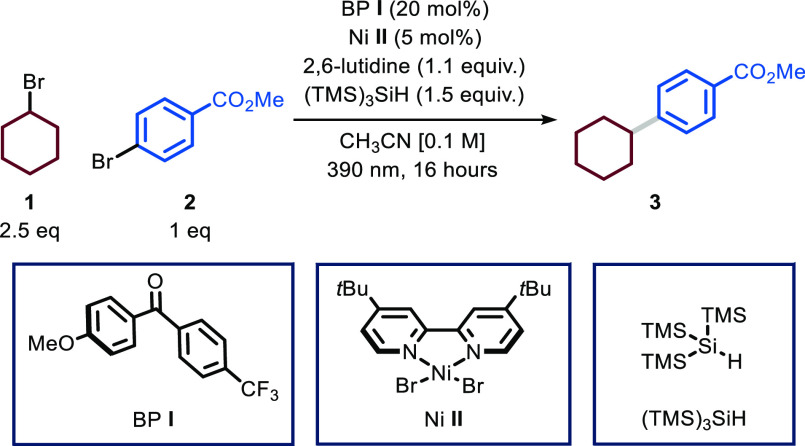
Table 1. Optimization of the Reaction Conditions.
| entry | deviation | 3 yield (%)a,b |
|---|---|---|
| 1 | none | 75 (72) |
| 2 | 1.5 equiv of 1 | 51 |
| 3 | 1.1 equiv of (TMS)3SiH | 60 |
| 4 | Ph3SiH instead of (TMS)3SiH | 20 |
| 5 | acetone in place of CH3CN | 67 |
| 6 | Vapourtec 365 nm 45 min | 74 |
| 7 | no I, II, 2,6-lutidine or (TMS)3SiH | |
| 8 | no light or no light at 60 °C |
1 (1.25 mmol), 2 (0.5 mmol), BP I (20 mol %), Ni II (5 mol %), 2,6-lutidine (0.55 mmol), (TMS)3SiH (0.75 mmol) at room temperature, 390 nm for 16 h.
NMR yields using trichloroethylene as an external standard. Yield of the isolated compound is given in parentheses.