Abstract
The evidence base concerning the management of benign pleural effusions has lagged behind that of malignant pleural effusions in which recent randomised trials are now informing current clinical practice and international guidelines.
The causes of benign pleural effusions are broad, heterogenous and patients may benefit from individualised management targeted at both treating the underlying disease process and direct management of the fluid. Pleural effusions are very common in a number of non-malignant pathologies, such as decompensated heart failure, and following coronary artery bypass grafting. Pleural fluid analysis forms an important basis of the diagnostic evaluation, and more specific assays and imaging modalities are helpful in specific subpopulations.
Options for management beyond treatment of the underlying disorder, whenever possible, include therapeutically aspirating the fluid, talc pleurodesis and insertion of an indwelling pleural catheter. Randomised trials will inform clinicians in the future as to the risks and benefits of these options providing a guide as to how best to manage patient symptoms in this challenging clinical setting.
Short abstract
Benign pleural effusion management is challenging and based on limited evidence. Treatment options are discussed http://ow.ly/10EOSN
Introduction
The management of malignant pleural effusions has seen considerable progress within the past decade, with a number of randomised controlled trials now informing practice [1–3]. This progress, by contrast, has not been mirrored in non-malignant pleural disease, which has an evidence base still formed largely of observational studies. As a result, the management of non-malignant pleural effusions is often guided by techniques established and validated in malignant disease. Similarly, though international guidelines produced by the British Thoracic Society and American Thoracic Society are available for malignant pleural effusions [4, 5], no such equivalent guidelines exist for non-malignant pleural disease.
The potential causes of non-malignant pleural effusions are numerous, often uncommon and in some cases poorly understood. This lack of clear classification and definition may, in part, explain the absence of high-quality evidence and established guidelines. As affected patients frequently have comorbidities and their pleural effusions may be a manifestation of a disease process managed by non-respiratory specialists, close multidisciplinary links are particularly important in this patient group to ensure coordinated and optimised care pathways.
The purpose of this article is to outline the burden and spectrum of non-malignant pleural effusions and to summarise the evidence base for management strategies.
Epidemiology
Non-malignant pleural effusions are common and the spectrum of disease is broad. In particular, pleural effusions related to heart failure are frequently present in adult patients admitted to hospital and those in critical care settings. Many of these effusions may be subclinical and resolve in parallel with treatment of the underlying disease process. However, there is a substantial burden of symptomatic disease requiring dedicated treatment.
Unpublished audit data from Bristol (UK) of 327 patients with non-malignant effusions referred to a tertiary pleural service for further investigation over a 5-year period are shown in table 1. This data demonstrates the distribution of possible aetiologies after effusions caused by malignancy or trauma have been excluded.
TABLE 1.
Proportions of causes of non-malignant pleural effusions
| Condition | Patients |
| Pleural infection# | 131 (40.0%) |
| Congestive cardiac failure | 81 (34.8%) |
| Idiopathic pleuritis/undiagnosed | 41 (12.5%) |
| Benign asbestos pleural effusion | 27 (8.3%) |
| Liver cirrhosis | 13 (4.0%) |
| Renal failure | 10 (3.1%) |
| Pulmonary embolism | 6 (1.8%) |
| Post-coronary artery bypass graft | 4 (1.2%) |
| Drug reaction | 3 (0.9%) |
| Rheumatoid effusion | 3 (0.9%) |
| Trapped lung | 2 (0.6%) |
| Pancreatitis | 2 (0.6%) |
| Other¶ | 4 (1.2%) |
#: e.g. parapneumonic effusions, empyema or tuberculous pleural effusions; ¶: e.g. peritoneal dialysis, systemic lupus erythematosus or a result of paint inhalation.
Evaluation of a pleural effusion
The evaluation of pleural disease relies upon a careful history and examination but will frequently necessitate imaging and pleural fluid analysis in order to narrow the differential diagnosis.
The most common symptom resulting from a pleural effusion is breathlessness. The extent of breathlessness may be disproportionate to the size of the effusion and influenced by coexistent cardiac and lung diseases. Dyspnoea may result from a reduction in normal diaphragmatic movement in addition to the reduction in tidal volume directly associated with the mass effect of fluid within the thoracic cavity [6].
The speed of onset of symptoms (most typically breathlessness) will frequently provide a steer to the probable underlying cause, although accompanying radiology may provide an objective assessment of this. Table 2 details common features in the patient's history that may be relevant.
TABLE 2.
History, pleural fluid assays and other investigations used in the evaluation of non-malignant pleural effusions
| Category | Details |
| History | |
| Presenting symptoms | Speed of onset |
| Other respiratory symptoms | |
| Associated multisystem symptoms | |
| Symptoms of bacterial or viral infection | |
| Symptoms of connective tissue disease | |
| Past medical history | Previous thoracic surgery |
| Heart failure | |
| Liver cirrhosis | |
| Renal failure | |
| Connective tissue disease (e.g. rheumatoid arthritis) | |
| Lymphangioleiomyomatosis | |
| IgG4-related disease | |
| History of pleural infection | |
| Drug history | Medications associated with pleural disease |
| Immunomodulatory treatment | |
| Ovarian hyperstimulation | |
| Social history | Cigarette smoking |
| Travel history | |
| Occupational history | Asbestos exposure |
| Other industrial exposure (e.g. beryllium) [7] | |
| Inhaled allergens | |
| Investigations | |
| Standard pleural assays | Protein |
| Lactate dehydrogenase | |
| Glucose | |
| Differential cell count | |
| Advanced pleural assays | Amylase |
| Triglycerides | |
| Cholesterol | |
| Microscopy for chylomicrons | |
| Adenosine deaminase | |
| Creatinine | |
| N-terminal pro-brain natriuretic peptide# | |
| Other investigations | Full blood count |
| Urea and electrolytes | |
| Liver function | |
| Inflammatory markers | |
| Autoimmune screen | |
| N-terminal pro-brain natriuretic peptide | |
| Urine analysis |
#: serum levels correlate closely with pleural fluid levels; therefore, measurement of pleural fluid levels is usually unnecessary.
Pleural fluid analysis
Pleural fluid analysis will often provide crucial information in the evaluation of a non-malignant pleural effusion, in addition to helping establish whether further investigations for malignancy are required. Table 2 details the components of standard testing of pleural fluid and blood and those more targeted assays, which may be indicated in specific cases of suspected non-malignant effusions.
Factors dictating the use of advanced tests may arise from the appearance of the fluid or patient factors specific to the case. Fluid with a milky appearance, for instance, increases the possibility of a chylothorax and should be evaluated with a request for triglyceride and cholesterol levels and the presence or absence of chylomicrons. Allowing the fluid to stand on a bench for 30 min can also be helpful in differentiating empyema from chylothorax, as a clear supernatant will develop in empyema whereas a chylous effusion will remain uniformly cloudy.
Although the criteria of Light is not completely specific or sensitive for the identification of a malignancy, an effusion classified as a transudate by this criteria is strongly suggestive of a non-malignant cause and specifically raises the possibility of an effusion due to heart failure, renal failure or a hepatic hydrothorax [8]. It should, however, be noted that ∼5% of malignant pleural effusions are initially classified as a transudate according to the criteria of Light [9].
Pleural infection is typically indicated by low pH or low glucose, the values of which are correlated [10]. However, these fluid characteristics may also be seen in effusions due to rheumatoid arthritis, advanced malignancy and oesophageal perforation [11].
Differential cell counts are not universally tested in all laboratories, but provide useful information. Pleural effusions are commonly lymphocyte predominant, though the presence of significant numbers of neutrophils and eosinophils may help narrow the possible diagnoses. Typically, chronic pleural effusions are predominantly occupied by lymphocytes, and those due to more acute processes with neutrophils. The presence of eosinophils is often associated with air or blood in the pleural space, but other diagnostic possibilities remain [12].
Standard blood biochemistry for a pleural effusion is likely to consist of a full blood count, urea and electrolytes, liver function, coagulation assays and C-reactive protein. More specific tests including N-terminal pro-brain natriuretic peptide (NT-proBNP) and assays such as a rheumatoid factor, autoimmune profile and anti-neutrophil cytoplasmic antibody may be indicated in specific cases.
In many cases of non-malignant effusions, the diagnosis may be clear following an initial assessment and the examination of pleural fluid, though in a substantial number of patients more detailed investigations may be required.
Imaging
Chest radiographs and thoracic ultrasound play key roles in the evaluation and management of pleural effusions [13]. Pleural appearances seen on computed tomography (CT) in patients with non-malignant disease may be more subtle than in those with malignant findings. In the quantification and characterisation of pleural effusions and pleural thickening, CT is, however, an invaluable tool. The value of using late venous phase intravenous contrast has been demonstrated in characterising pleural thickening and identifying the presence of malignant pleural disease [14]. Pleural fluid aspiration or drainage prior to CT imaging has been shown to not significantly improve diagnostic performance in the evaluation of pleural pathology [15].
Magnetic resonance imaging (MRI) does not currently have a routine role in the evaluation of pleural disease but provides high-quality imaging of chest wall invasion in malignant disease, which is reflected in its use for malignant mesothelioma. It may be useful in younger patients and in patients in whom chest wall soft tissue abnormalities are suspected. MRI has been demonstrated to aid characterisation of pleural effusions as transudates and simple or complex exudates [16]. MRI scanning and lymphoscintigrams can be very useful in demonstrating the site of the leak from the lymphatic system in cases of chylothorax [17].
Tissue diagnosis
Pleural biopsy may be achieved as a closed procedure, in the case of radiologically guided percutaneous biopsy, or performed under direct visual guidance in the case of thoracoscopy. The sensitivity of CT-guided biopsy in establishing pleural malignancy has been shown to be superior to Abrams' biopsy (87% versus 47%) [18], though in regions with a high prevalence of tuberculosis Abrams' biopsies may still have a role in view of the greater sensitivity they exhibit in the diagnosis of tuberculosis (82%) [19].
Thoracoscopic procedures may range from a single-port procedure undertaken with local anaesthetic and sedation to multiple port video-assisted thoracoscopic surgery (VATS) under general anaesthetic. The question as to which of these procedures should be undertaken may depend on local availability and patient factors, which may suggest a more or less invasive approach being favoured.
The rationale for performing a biopsy is usually to specifically exclude malignancy, although they may also be useful in establishing certain non-malignant diseases. The histopathological features of many non-malignant effusions are typically nonspecific and a biopsy in isolation is unlikely to definitively establish aetiology. However, notable exceptions exist, such as tuberculosis, which could be established beyond doubt if mycobacteria are identified on pleural biopsies.
Effusion pathology
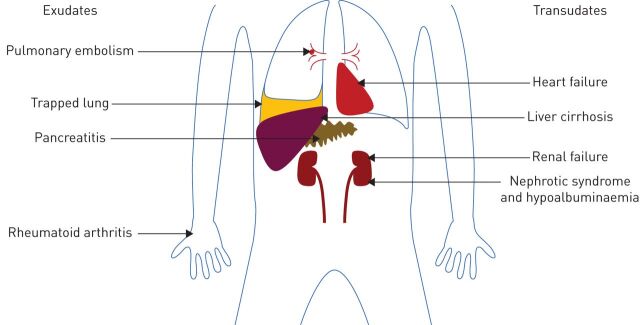
Pleural effusions are caused by a wide array of processes, a number of which are discussed below. Figure 1 demonstrates the multiple conditions and organ systems potentially involved in the development of a non-malignant effusion, and their characteristic classification by Light's criteria.
FIGURE 1.
Schematic diagram illustrating a range of multisystem pathologies leading to non-malignant effusions, categorised by their tendency to cause exudates or transudates.
Pleural effusions develop due to an increase in production or a reduction in reabsorption of pleural fluid or both processes in unison.
Cardiac
Heart failure leads to pleural effusion formation due to an increase in pulmonary capillary pressure and a consequent leak into the pleural space. This arises as a result of a range of cardiac pathology including left ventricular dysfunction, which may be diastolic or systolic, valve dysfunction or constrictive pericarditis. The prevalence of clinically identified heart failure is ∼3–20 cases per 1000 population and increases markedly with age [20]. Of those patients with decompensated heart failure requiring diuretic treatment, 87% have pleural effusions on CT [21]. Patients with uncomplicated heart failure with pleural effusions have bilateral effusions in 73% of cases [22]. A large proportion of these pleural effusions will improve with optimised treatment for heart failure. A prospective study of 60 patients demonstrated that 89% of those patients with an initial response to diuretic treatment no longer had a pleural effusion after 2 weeks of follow-up [21]. Treatment typically involves medication, such as diuretics, angiotensin converting enzyme inhibition, β-blockade, or procedures such as cardiac resynchronisation or intervention to treat valve dysfunction. In recent decades considerable advances have been made in the management of heart failure, resulting in a shift away from patients presenting with acute decompensated disease towards a more stable and chronic course.
Although more commonly associated with left-sided failure, pleural effusion also occurs in right-sided heart failure. Though they are most commonly small effusions, ∼13% of patients with idiopathic or familial pulmonary artery hypertension have pleural effusions attributed to right-sided heart failure [23]. The process of accumulation of the fluid is thought to be due to processes including decreased pleural fluid reabsorption, increased hydrostatic pressure in bronchial veins, or dilated pulmonary arteries obstructing lymphatic flow. These effusions appear to be most commonly right sided (58%) or bilateral (26%), and are typically transudates [23]. Patients with cardiac effusions treated with diuretics are reported to have an increased pleural fluid protein level relative to its blood level, resulting in the effusion being classified as an exudate by Light's criteria [24]. In patients with an exudative effusion suspected of having a cardiac cause, correlation with echocardiography or NT-proBNP measurement may be helpful to clarify the aetiology; care should be taken to exclude other relevant potential causes, such as malignancy or pulmonary emboli.
Direct management of pleural effusions due to heart failure may be hampered by the presence of bilateral effusions. However, despite isolated right- or left-sided effusions being uncommon, bilateral effusions frequently have a larger left- or right-sided component [22]. In view of the dyspnoea associated with heart failure itself, patients may benefit considerably from drainage of effusions that persist despite optimised heart failure treatment.
Pleural effusions are present in nearly all patients immediately after coronary artery bypass grafting and diminish over time, with the majority of early effusions remaining small and not a cause of significant symptoms [25, 26]. Pleural effusions following coronary artery bypass grafting may be categorised as early or late effusions, which are thought to be distinct processes, with early effusions more likely to be haemorrhagic, eosinophil predominant and associated with a high LDH level, and later effusions more likely to be non-haemorrhagic and characterised by a lymphocyte predominant, inflammatory-mediated process [27].
Of those patients with a persistent pleural effusion after coronary artery bypass grafting, many only require a single thoracentesis with more long-term treatment rarely required [28]. Pleural effusions also occur as a manifestation of post-cardiac injury (or Dressler's) syndrome [29].
Hepatic
Hepatic hydrothorax is present in ∼5% of patients with liver cirrhosis and ascites [30]. Hepatic hydrothoraces are most commonly right sided, although they may be bilateral or exist solely on the left [31]. This tendency towards the right side is thought to be due to a predisposition of diaphragmatic defects affecting the right hemidiaphragm [32]. In the presence of such a defect, the negative intrathoracic pressure results in a flow of fluid from the abdomen to the chest.
Although less common, hepatic hydrothorax also occurs in the absence of clinically apparent ascites [31]. However, even in these patients a one-way flow of radiolabelled colloid across the diaphragm has been demonstrated, suggesting that in these patients flow across the diaphragm matches the rate of ascites formation [33].
The presence of hepatic hydrothorax in liver cirrhosis is associated with a particularly poor prognosis. In a study of 77 patients with hepatic hydrothorax, only 33 (43%) survived for 1 year after diagnosis [31].
Hepatic hydrothorax is managed with salt restriction and diuretic therapy, and treatment of ascites may result in an improvement in the quantity of fluid above the diaphragm. Surgical repair of the diaphragm is used, though more commonly, in the presence of persisting fluid, a trans-jugular intrahepatic portosystemic shunt or liver transplant may be needed. If these procedures are ineffective, unavailable or contraindicated, direct removal of pleural fluid may be required. Additionally, some centres are using indwelling pleural catheters as a bridge to more definitive management, such as liver transplantation.
Fluid and protein loss is a concern that this particularly pertinent to patients with pleural effusions due to hepatic hydrothorax and has given rise to the practice of administering intravenous human albumin solution, as is used in patients with cirrhosis requiring paracentesis. Though no clear guidance or evidence exists, use of human albumin solution is frequently advocated by hepatologists for patients with hepatic hydrothorax, in whom the typically larger volume of fluid drained via an indwelling pleural catheter may be a particular problem [34].
Renal
In a retrospective review of a cohort of 257 patients receiving long-term haemodialysis for renal failure, 52 (20%) patients had a history of pleural effusion [35]. These pleural effusions were most commonly due to hypervolaemia (62%) and typically associated with bilateral transudative effusions. The remainder were attributed to heart failure, uraemia or infection. An observational study of 430 patients with chronic kidney disease (stage 3–5) established the incidence of pleural effusions to be 6.7%, substantially lower than in patients with more severe disease necessitating renal replacement therapy [36].
Pleural effusions attributed to renal failure are typically bilateral and classified as transudates by Light's criteria. Of those patients who develop an exudative pleural effusion on long-term haemodialysis, the most common cause is uraemic pleuritis, which may be unilateral and bilateral and develops in ∼4% of patients on long-term dialysis. Uraemic pleuritis is typically associated with a blood stained effusion and biopsies indicate fibrinous pleuritis [35].
Patients on long-term renal replacement therapy are likely to be more vulnerable to pleural infection given the potential for a degree of immunodeficiency seen in these patients. The need to carefully exclude the possibility of pleural infection is therefore an important consideration in this patient group.
Peritoneal dialysis represents an alternative process through which a pleural effusion may accumulate in patients with renal failure [37]. This complication is reported to affect 1.6% of patients receiving chronic ambulatory peritoneal dialysis and may be indicated by a reduction in the adequacy of peritoneal dialysis. The development of pleural effusion may occur around the time of peritoneal dialysis initiation or much later; 50% of effusions arise within 30 days of peritoneal dialysis initiation [38]. The biochemistry of pleural fluid in such cases will characteristically represent the constituents of the peritoneal dialysate, typically with very low protein and lactate dehydrogenase. Similar to the pleural fluid that accumulates in liver cirrhosis due to diaphragmatic transit of ascites, right-sided pleural effusions due to peritoneal dialysis are much more common. Recommended treatment options for pleural effusions due to peritoneal dialysis consist of conservative measures, such as a temporary switch to haemodialysis, the use of more frequent and smaller volume exchanges, chemical pleurodesis or surgical VATS [39].
Other causes
A wide variety of less common pathologies may cause a pleural effusion, the diagnosis of which may be substantially less straightforward than those caused by heart, liver or renal failure, the underlying organ failure of which is likely to be recognised prior to the development of the pleural effusion.
Benign asbestos pleural effusion is a diagnosis of exclusion, with the possibility of mesothelioma needing careful consideration, as it does in all patients with a history of asbestos exposure and pleural disease. Benign asbestos pleural effusion is usually unilateral and characterised by an exudative effusion typically occurring within 15 years of asbestos exposure, but may arise much later [40]. Effusions may be asymptomatic or associated with breathlessness and chest pain and in around one-third of cases high levels of eosinophils are present within the fluid [41]. Pleural biopsies are required to exclude malignancy, and radiological follow-up over a 2-year period with chest CT is recommended in view of the need to establish whether there is progressive abnormality and pleural thickening. Benign asbestos pleural effusion is frequently associated with a transient effusion, for which long-term management may not be required.
Roughly 50% of patients with yellow nail syndrome have pleural effusions alongside lymphoedema, chronic respiratory manifestations and the nail abnormalities themselves [42]. Pleural fluid analysis will typically demonstrate a lymphocytic effusion, though some patients may develop a chylothorax. Biopsies are likely to reveal a chronic fibrosing pleuritis [42].
Pulmonary embolism
Pleural effusions are a frequent finding in as many as 30% of patients with pulmonary embolism and are thought to be due to ischaemia itself or cytokine release [43]. These effusions are, however, typically present for no more than 1 month [44], and 90% are smaller than one-third of the hemithorax [45]. A degree of breathlessness disproportionate to the size of the pleural effusion and pleuritic chest pain may be a feature of effusions associated with pulmonary embolism.
Though pleural effusions are typically present on the same side as the lung affected by pulmonary embolism, they may be unilateral despite bilateral embolic disease or bilateral when the pulmonary embolism is unilateral. Surprisingly, the effusion is also present unilaterally and contralateral to the embolism in ∼7.5% of patients with effusions due to pulmonary embolism [45].
Earlier studies with methodological limitations suggested that pleural effusions due to pulmonary emboli may be transudates; however, more recent series have established that all pulmonary embolism-associated effusions are exudates according to Light's criteria [45]. Additionally pleural fluid is typically blood stained and characteristically neutrophil predominant [45]. As these effusions are typically very small, a larger effusion should potentially prompt evaluation for a concomitant cause.
Pancreatitis
An elevated pleural fluid amylase, indicated by either a pleural fluid amylase greater than serum amylase or a pleural fluid amylase above the upper limit of normal for the serum value, is suggestive of acute or chronic pancreatitis. Rupture of a pancreatic pseudocyst and fistula formation may result in direct communication with the thoracic cavity and accumulation of pleural fluid.
Elevated pleural fluid amylase is also seen in effusions due to oesophageal rupture and possibly also in malignant pleural effusions. Isoenzyme analysis may allow a distinction to be made between salivary and pancreatic amylase in difficult cases.
Autoinflammatory
Rheumatoid arthritis is associated with a variety of pulmonary and pleural manifestations. A high rheumatoid factor titre is predictive of pleural effusion alongside other extra-articular features. Rheumatoid pleural disease is more common in men [46]. Though pleural effusions are described in 3–5% of patients with rheumatoid arthritis, evidence of abnormalities on chest radiographs are much more common [47]. Pleural effusions associated with rheumatoid arthritis are typically painless, characterised as exudates and have a low pH and glucose and may, therefore, be confused with pleural infection.
In patients with rheumatoid arthritis presenting with a pleural effusion, the possibility of medications causing the effusion should be considered as a number of anti-rheumatoid therapies including methotrexate, sulfasalazine and D-Penicillamine may be potentially implicated.
Systemic lupus erythematosus may give rise to pleural effusions directly through lupus pleuritis or through a secondary process such as renal disease, heart failure or pulmonary embolism [47]. Pleural effusions caused by lupus typically have a higher glucose level compared with those associated with rheumatoid arthritis and may cause significant chest pain as part of the serositis syndrome. High anti-nuclear antibody titres are sensitive but not specific for a lupus effusion; high antinuclear antibody titres may also be seen in malignant pleural effusions [48]. However, lupus erythematosus cells are considered to be highly specific.
Connective tissue diseases such as systemic sclerosis, mixed connective tissue disease, granulomatosis with polyangiitis, ankylosing spondylitis and Still's disease may also have features of pleural involvement. Familial Mediterranean fever may cause pleural inflammation associated with unilateral pleuritic chest pain and pleural effusions, which are most commonly small and short lived.
Chylothorax
A chylothorax may arise as a result of disruption of the thoracic duct in its path from the cisterna chyli up the posterior mediastinum to the left subclavian vein. An interruption in the flow of chyle results in the accumulation of fluid within the thoracic cavity. As the thoracic duct crosses the midline, a chylothorax may be present in either hemithorax or bilaterally.
Around half of chylothoraces are due to trauma. Of the remainder, 38% are due to malignancy, most commonly lymphoma or leukaemia. Non-malignant causes include congenital lymphatic disorders such as Gorham or Milroy disease, or conditions such as lymphangioma, lymphangioleiomyomatosis, yellow nail syndrome or lupus, or may be secondary to chylous ascites, most often a complication of liver cirrhosis [49].
Chylothoraces represent a different pathological mechanism to those shared by most other non-malignant effusions, and warrant specific consideration. The diagnosis of a chylothorax is often made on the basis of the typical appearance of fluid and easily established on the basis of pleural fluid biochemistry or the presence of chylomicrons, but in some instances the appearance may be mistaken for a purulent effusion due to infection. A reduced fat diet, if carefully adhered to, may reduce flow of chyle and result in spontaneous closure of the thoracic duct defect. If unsuccessful, surgical ligation or percutaneous embolisation approaches may be appropriate to directly block the chyle leak. Lymphoscintigraphy may be helpful in the identification of the site of leak (figure 2).
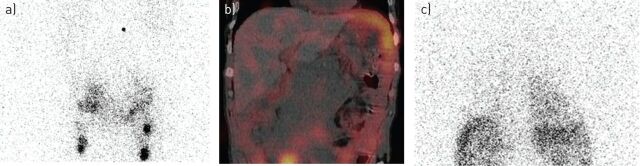
FIGURE 2.
Lymphoscintigram and SPECT-CT (single photon emission computer tomography combined with integrated low-dose computed tomography) imaging in a patient with a chylothorax. A 74-year-old man with non-Hodgkin lymphoma had recurrence of a right-sided pleural effusion following previous talc pleurodesis. An intercostal catheter was inserted and drained >3.5 L of milky fluid with a triglyceride level of 17 mmol·L−1, confirming a chylothorax. Chyle flow decreased but continued despite total parental nutrition. Computed tomography of the abdomen also indicated a confluent lymphomatous mass around the aorta and inferior vena cava with ascites and lymphadenopathy. A lymphoscintigraphy was performed to determine the point of chyle leakage. Tc99-labelled human serum albumin-DTPA was injected into the first pedal interdigital web space bilaterally and serial whole body SPECT-CT was performed at intervals between 90 min and 6.5 h following tracer administration. This case illustrates the importance of Tc99 lymphangioscintigraphy as it identified an abdominal chyle leak site for the right chylothorax (instead of leakage at the thoracic portion of the thoracic duct), and changed the management of the patient. The patient was referred for radiotherapy to the abdominal mass. a) Lymphoscintigram showing a site of chyle leak in the abdomen at 2 h following injection. b) Low dose SPECT-CT showed pooling of the tracer below the diaphragm confirming chyle leak from the abdominal source into the abdomen. c) Follow-up lymphoscintigram showed tracer uptake over the right pleural cavity, confirming that chyle leak from the abdomen migrated transdiaphragmatically causing the right chylothorax at 6.5 h after injection. (Images courtesy of A. Yogendran, University of Western Australia, Perth, Australia).
Cholesterol effusions (or pseudochylous effusions) may have a similar milky appearance to a true chylous effusion, but may be distinguished following the measurement of triglyceride and cholesterol levels. The two most common causes of cholesterol effusions are tuberculosis and rheumatoid pleural disease; helminth infections and cases secondary to malignancy occur less commonly [50].
Trapped lung
Trapped (unexpandable) lung may cause a pleural effusion through the development of a greater negative pleural pressure, caused by visceral pleural thickening preventing lung expansion [51]. This negative pressure facilitates movement from pleural capillaries into the pleural space. Frequently, the development of trapped lung is a consequence of a pathological process already giving rise to a pleural effusion, after which visceral pleural thickening perpetuates the effusion [52]. This process can occur in the context of a long-standing effusion of any cause. However, a history of past pleural infection, thoracic surgery, rheumatoid pleural disease, thoracic radiation or a haemothorax are described as potential causes [52].
Iatrogenic
Pleural effusions caused by medication are uncommon, though a wide range of medications may potentially be implicated. More than 70 drugs have been reported to cause pleural effusions, and among them are cardiovascular medications and ergoline drugs, as well as chemotherapy agents [53]. Drugs more commonly associated include methotrexate, amiodarone and nitrofurantoin [13]. Mechanisms include hypersensitivity, direct toxicity, free radical production and chemical-induced inflammation [54]. A number of drugs may cause an effusion through lupus pleuritis. Pleural fluid eosinophilia may alert the clinician to the possibility of a drug-induced effusion, though this finding is neither sensitive nor specific. In addition to drug causes, iatrogenic pleural effusions may also arise as a complication of therapeutic interventions such as radiotherapy.
Pleural fluid may, in some cases, be exogenous fluid that accumulates within the pleural space, due to either transit of fluid from another body cavity, such as in peritoneal dialysis or an iatrogenic consequence of misplaced central venous catheters, oesophageal perforation, or in the case of spinal or urological surgery an accumulation of cerebrospinal fluid or urine, respectively, within the pleural space. The management of these processes will not be discussed here.
Treatment of non-malignant pleural effusions
The management of non-malignant pleural effusions is predominantly focussed on relief of symptoms. The accumulation of a pleural effusion may significantly increase levels of dyspnoea in a patient with a considerable pre-existing symptom burden from comorbid conditions. Non-malignant effusions, in and of themselves, do not represent a significant mortality risk, but are the cause of substantial morbidity, the adequate treatment of which relies on identification of the underlying disease.
Treatment of underlying aetiology
Treatment targeted at the underlying cause for pleural effusion accumulation is crucial. Such treatment may attenuate or lead to complete resolution of the effusion. Additionally, in many cases such treatment may alleviate symptoms or improve prognosis independently of the pleural effusion.
It is beyond the scope of this article to address the direct treatment of all potential pathologies that lead to pleural effusions; therefore, the focus here will be on direct treatment of the effusion itself. In substantial numbers of patients, direct intervention either to remove pleural fluid or to prevent its accumulation may be required.
Targeted pleural fluid management
Pleural fluid aspiration may be required prior to definitively establishing an aetiology, and in other patients is a consideration after a diagnosis has been confirmed. In a small number of patients, despite best efforts, it may not be possible to reach a clear diagnosis and in others it may be that treatment targeted at addressing symptoms may be a pragmatic approach preferred over and above ongoing and potentially more invasive investigations, particularly in patients with a poor prognosis. Interestingly, the removal of pleural fluid appears to result in an improvement in breathlessness not mirrored directly by the degree of improvement in gas exchange [55].
The management options used for patients with non-malignant pleural effusions are closely aligned to those used in patients with malignant disease, in whom the evidence base for the spectrum of available treatments is frequently stronger.
Some non-malignant pleural effusion may require observation alone. Small and asymptomatic pleural effusions occurring shortly after cardiac surgery for instance may well resolve spontaneously and any non-malignant effusion reaching a steady state without causing symptoms should not require intervention.
Therapeutic aspiration
Therapeutic aspiration fulfils an important role in the management of any pleural effusion. At the time of obtaining a pleural fluid sample for diagnostic purposes, a substantial volume may be removed for therapeutic purposes. This should improve patient symptoms during the period in which diagnostic evaluation may occur, allows evaluation of the speed of fluid build-up, provides evidence of the degree of symptomatic improvement that can be gained through removal of fluid, and clarifies whether there is any evidence of trapped lung which may direct future management.
In some patients, therapeutic aspiration may represent a legitimate therapeutic option in the long term. Depending on the speed at which fluid re-accumulates, therapeutic aspirations may only be required at a frequency acceptable to both clinicians and patients. The downside to this approach is that symptoms may progressively worsen while fluid accumulates. As this strategy may be administered as an outpatient and with non-specialist equipment, it has little financial cost over and above that of the patient and clinician's time. However, the cumulative risk of bleeding and the introduction of infection during repeated procedures should be considered. Though the risk of trapped lung developing in patients requiring repeated therapeutic aspirations is uncertain, the possibility of the visceral pleura becoming thickened over time as it is chronically bathed in pleural fluid is a potential disadvantage of this approach.
In malignant pleural effusions, a maximal volume of aspiration of 1.5 L is advised [4]. Though the risk of re-expansion pulmonary oedema is low, caution should be advised when considering larger volume aspirations and the mechanism causing re-expansion oedema also applies in non-malignant effusions. Some authorities recommend the use of manometry as a guide to predict the appropriate volume to remove [56]. In the absence of manometry then an appropriate strategy may be to aspirate only 1.5 L on a single occasion, though if the patient experiences no symptoms such as cough or chest pain, it may be reasonable to cautiously aspirate more than this volume on a case-by-case basis.
Indwelling pleural catheters
The PleurX catheter (CareFusion, Vernon Hills, IL, USA) was approved by US Food and Drug Administration in 1997 for the management of malignant pleural effusions [57]. Since 2001, the license has extended to additionally apply to non-malignant pleural effusions [58].
Indwelling pleural catheter use in malignant pleural effusions. Increasing evidence is now available supporting the safe use of indwelling pleural catheters in malignant pleural disease, which allows outpatient-based treatment. Use of indwelling pleural catheters in malignant disease is considered to be cost-effective compared with talc pleurodesis (which necessitates an inpatient stay), though this is dependent on expected prognosis [59, 60].
The use of indwelling pleural catheters in non-malignant effusions is not as well supported by available evidence and a lack of familiarity with indwelling pleural catheters amongst the multidisciplinary team involved in the care of these patients may contribute to their use not being as widespread. Whether indwelling pleural catheters are cost-effective in non-malignant disease has not yet been established and neither has the question of which patients with non-malignant effusions are likely to benefit most.
There are a number of observational studies reporting on the use of indwelling pleural catheters in non-malignant disease, with the majority of studies including a range of patient groups rather than specific disease groups in isolation. Complication rates associated with the use of indwelling pleural catheters in non-malignant effusions appear similar to those seen in patients with malignant disease [34, 61]. A review of available literature including 377 patients included in observational studies consisted of a varied case mix, with largest contributions being those patients with congestive cardiac failure (40%), hepatic hydrothorax (17%) or idiopathic pleuritis (15%). The most common complications were empyema or drain site infection which occurred in 5.2% and 2.9%, respectively [61].
Pleurodesis may occur in patients treated with an indwelling pleural catheter without this being the express purpose (figure 3). Often described as a “spontaneous” pleurodesis, this process occurs in around one-third of patients with non-malignant effusions treated with an indwelling pleural catheter. The rate of pleurodesis appears variable and dependent on the cause. The rate of spontaneous pleurodesis appears to be particularly low in hepatic hydrothoraces [34].
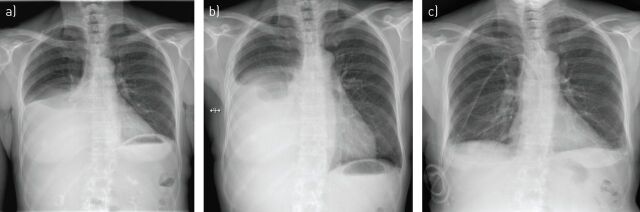
FIGURE 3.
Chest radiographs of a patient with a transudative pleural effusion due to biopsy confirmed systemic amyloidosis causing nephrotic syndrome. a) At presentation with right-sided pleural effusion. b) Recurrent pleural effusion following attempted talc pleurodesis which was performed due to frequent requirement for pleural aspiration. c) 6 months after placement of an indwelling pleural catheter. At this point a reduction in the volume of fluid drained indicated a “spontaneous” pleurodesis and allowed drain removal.
The use of indwelling pleural catheters in non-malignant effusions is the subject of a UK-based randomised controlled trial (REDUCE), recruitment to which is ongoing (www.isrctn.co.uk ISRCTN66354436). This study involves patients with symptomatic pleural effusions with heart failure or liver failure which persist despite optimised treatment being randomised to either treatment with an indwelling pleural catheter or therapeutic pleural aspirations as required. This study should, upon completion, provide robust data regarding the risks and benefits and cost-effectiveness of indwelling pleural catheters in this population.
Pleurodesis
Pleurodesis is a long established, safe and effective technique validated in patients with malignant effusion. As with the other techniques, there is less evidence to support its use in non-malignant effusions. A wide array of pleurodesis agents have been used in non-malignant effusions, though talc is the best described. Talc may be delivered via a chest drain (as a slurry) or at thoracoscopy (as poudrage).
There are historical concerns regarding talc containing traces of asbestos and reports of acute respiratory distress syndrome associated with the use of non-graded talc, which may be pertinent in patients with non-malignant disease. However, the use of contemporary French-graded talc allows these risks to be mitigated [62]. Pleurodesis with ungraded talc with small particles (<10 µM) has been shown to be associated with significantly greater levels of systemic inflammation and poorer gas exchange than graded talc in which these small particles are removed [63].
A large retrospective study of 611 patients which evaluated the success of talc pleurodesis via chest drain reported a success rate of 77% in the 68 patients with non-malignant causes. This study, however, did exclude patients undergoing more than one attempt at pleurodesis and the specific aetiologies amongst the included non-malignant effusions is not clear [64]. Glazer et al. [65] reported a success rate of 75% with talc pleurodesis in 16 non-malignant effusions of varying aetiology. A similar success rate of 80% was reported by Sudduth et al. [66] using a range of pleurodesis agents in 25 effusions with non-malignant cause.
There is evidence from a small case series suggesting that the use of talc pleurodesis for patients with hepatic hydrothorax is of considerable risk; the peri-procedure mortality is reported at 45.5% [67]. It is unclear to what extent this result may be explained by an extremely comorbid patient population.
In patients with hepatic hydrothorax undergoing talc poudrage at thoracoscopy a success rate of only 47.6% was reported in a small case series of 21 procedures in 18 patients [68]. As was the case in patients with hepatic hydrothorax receiving talc slurry pleurodesis via a chest tube, there were high rates of mortality (38.9% mortality during the 3 month follow-up) as well as considerable morbidity and long inpatient hospital stays.
Pleural-peritoneal and pleuro-venous shunts
Pleuro-peritoneal or pleuro-venous shunting of pleural fluid from the pleural space is a conceptually attractive idea. A case series of 12 patients with non-malignant effusions treated with a shunt from the pleural space to the subclavian or jugular vein reported promising results. The devices used consisted of a Denver Shunt with an external manual pump and all devices remained patent other than in one patient who experienced shunt occlusion at 4 weeks [69]. No air embolism was reported in this study, though this mechanism would result in a pneumothorax or pneumothorax ex vacuo thus becoming a life-threatening complication. Pleuro-venous shunting would mitigate against the protein and volume loss related to external drainage of fluid, which hepatic hydrothorax patients may be particularly susceptible to.
Pleuro-peritoneal devices have been used successfully with evidence of effective symptomatic relief in both malignant and non-malignant pleural effusions though are typically contraindicated in patients with ascites [70–72]. In view of the pressure difference between the pleural and peritoneal space a pump is required to achieve flow, and devices used have incorporated a unidirectional valve.
Surgical techniques
The frailty and comorbidity of some patients with non-malignant pleural effusion may act as a deterrent against some more invasive techniques, but surgical pleurectomy may be an appropriate option in some patients with a persistent effusion if less invasive techniques have been attempted and failed, or are otherwise contraindicated.
Future directions
As life expectancies rise and the prevalence of comorbidity increases, an increase in the prevalence of non-malignant pleural effusions should be expected. Treatments validated in malignant pleural disease cannot be assumed to be effective in non-malignant disease and equally, strategies effective in the treatment of a rheumatoid pleural effusion may not be so in a hepatic hydrothorax. Variations in the accepted techniques make randomised studies difficult to establish, particularly in respect of the management representing standard care which in many cases is not universally accepted, or deemed appropriate across a range of specialist centres.
Despite this, there is an increasing body of observational data and randomised studies are ongoing which should provide high-quality evidence upon which to base practice. In the future, pleural disease guidelines should be structured to include guidance on non-malignant pleural effusions alongside guidance for the management of pleural infection and malignant pleural disease.
Conclusion
Non-malignant pleural effusions encompass a heterogenous group of multisystem pathologies. The evaluation of such patients ranges from the straightforward patient with clinically obvious heart failure, the management of whom may be clearly ascertained, through to complex disease processes, with a number of contributing processes, through which meticulous investigation may be required. Relative to many other areas of respiratory medicine, it is often said there is a lack of research in pleural disease and this is particularly evident in non-malignant pleural disease which currently suffers from a lack of high-quality data to guide management and future research should address this unmet need.
Footnotes
Previous articles in this series: No. 1: Psallidas I, Kalomenidis I, Porcel JM, et al. Malignant pleural effusion: from bench to bedside. Eur Respir Rev 2016; 25: 189–198. No. 2: Bhatnagar R, Corcoran JP, Maldonado F, et al. Advanced medical interventions in pleural disease. Eur Respir Rev 2016; 25: 199–213.
Conflict of interest: Disclosures can be found alongside the online version of this article at err.ersjournals.com
Provenance: Submitted article, peer reviewed.
References
- 1.Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307: 2383–2389. [DOI] [PubMed] [Google Scholar]
- 2.Dresler CM, Olak J, Herndon JE II, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005; 127: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDS and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the time1 randomized clinical trial. JAMA 2015; 314: 2641–2653. [DOI] [PubMed] [Google Scholar]
- 4.Roberts M, Neville E, Berrisford R, et al. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65: Suppl 2, ii32–ii40. [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000; 162: 1987–2001. [DOI] [PubMed] [Google Scholar]
- 6.Thomas R, Jenkins S, Eastwood P, et al. Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med 2015; 21: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handa T, Nagai S, Kitaichi M, et al. Long-term complications and prognosis of chronic beryllium disease. Sarcoidosis Vasc Diffuse Lung Dis 2009; 26: 24–31. [PubMed] [Google Scholar]
- 8.Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77: 507–513. [DOI] [PubMed] [Google Scholar]
- 9.Gonlugur TE, Gonlugur U. Transudates in malignancy: still a role for pleural fluid. Ann Acad Med Singapore 2008; 37: 760–763. [PubMed] [Google Scholar]
- 10.Potts DE, Taryle DA, Sahn SA. The glucose–pH relationship in parapneumonic effusions. Arch Intern Med 1978; 138: 1378–1380. [PubMed] [Google Scholar]
- 11.Good JT Jr, Taryle DA, Maulitz RM, et al. The diagnostic value of pleural fluid pH. Chest 1980; 78: 55–59. [DOI] [PubMed] [Google Scholar]
- 12.Adelman M, Albelda SM, Gottlieb J, et al. Diagnostic utility of pleural fluid eosinophilia. Am J Med 1984; 77: 915–920. [DOI] [PubMed] [Google Scholar]
- 13.Hooper C, Lee YCG, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65: Suppl 2, ii4–ii17. [DOI] [PubMed] [Google Scholar]
- 14.Traill ZC, Davies RJ, Gleeson FV. Thoracic computed tomography in patients with suspected malignant pleural effusions. Clin Radiol 2001; 56: 193–196. [DOI] [PubMed] [Google Scholar]
- 15.Corcoran J, Acton L, Ahmed A, et al. Draining pleural effusions before radiological imaging does not improve diagnostic capability. Eur Respir J 2015; 46: Suppl. 56, PA1828. [Google Scholar]
- 16.Davis SD, Henschke CI, Yankelevitz DF, et al. MR imaging of pleural effusions. J Comput Assist Tomogr 1990; 14: 192–198. [DOI] [PubMed] [Google Scholar]
- 17.Yu DX, Ma XX, Wang Q, et al. Morphological changes of the thoracic duct and accessory lymphatic channels in patients with chylothorax: detection with unenhanced magnetic resonance imaging. Eur Radiol 2013; 23: 702–711. [DOI] [PubMed] [Google Scholar]
- 18.Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003; 361: 1326–1330. [DOI] [PubMed] [Google Scholar]
- 19.Koegelenberg CF, Bolliger CT, Theron J, et al. Direct comparison of the diagnostic yield of ultrasound-assisted Abrams and Tru-Cut needle biopsies for pleural tuberculosis. Thorax 2010; 65: 857–862. [DOI] [PubMed] [Google Scholar]
- 20.McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart 2000; 83: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kataoka H. Pericardial and pleural effusions in decompensated chronic heart failure. Am Heart J 2000; 139: 918–923. [DOI] [PubMed] [Google Scholar]
- 22.Woodring JH. Distribution of pleural effusion in congestive heart failure: what is atypical? South Med J 2005; 98: 518–523. [DOI] [PubMed] [Google Scholar]
- 23.Tang KJ, Robbins IM, Light RW. Incidence of pleural effusions in idiopathic and familial pulmonary arterial hypertension patients. Chest 2009; 136: 688–693. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Candeira S, Fernandez C, Martin C, et al. Influence of diuretics on the concentration of proteins and other components of pleural transudates in patients with heart failure. Am J Med 2001; 110: 681–686. [DOI] [PubMed] [Google Scholar]
- 25.Vargas FS, Cukier A, Hueb W, et al. Relationship between pleural effusion and pericardial involvement after myocardial revascularization. Chest 1994; 105: 1748–1752. [DOI] [PubMed] [Google Scholar]
- 26.Light RW. Pleural effusions following cardiac injury and coronary artery bypass graft surgery. Semin Respir Crit Care Med 2001; 22: 657–664. [DOI] [PubMed] [Google Scholar]
- 27.Sadikot RT, Rogers JT, Cheng DS, et al. Pleural fluid characteristics of patients with symptomatic pleural effusion after coronary artery bypass graft surgery. Arch Intern Med 2000; 160: 2665–2668. [DOI] [PubMed] [Google Scholar]
- 28.Light RW, Rogers JT, Moyers JP, et al. Prevalence and clinical course of pleural effusions at 30 days after coronary artery and cardiac surgery. Am J Respir Crit Care Med 2002; 166: 1567–1571. [DOI] [PubMed] [Google Scholar]
- 29.Dressler W. The post-myocardial-infarction syndrome: a report on forty-four cases. AMA Arch Intern Med 1959; 103: 28–42. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman FL, Hidemura R, Peters RL, et al. Pathogenesis and treatment of hydrothorax complicating cirrhosis with ascites. Ann Intern Med 1966; 64: 341–351. [DOI] [PubMed] [Google Scholar]
- 31.Badillo R, Rockey DC. Hepatic hydrothorax: clinical features, management, and outcomes in 77 patients and review of the literature. Medicine (Baltimore) 2014; 93: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strauss RM, Boyer TD. Hepatic hydrothorax. Semin Liver Dis 1997; 17: 227–232. [DOI] [PubMed] [Google Scholar]
- 33.Rubinstein D, McInnes IE, Dudley FJ. Hepatic hydrothorax in the absence of clinical ascites: diagnosis and management. Gastroenterology 1985; 88: 188–191. [DOI] [PubMed] [Google Scholar]
- 34.Bhatnagar R, Reid ED, Corcoran JP, et al. Indwelling pleural catheters for non-malignant effusions: a multicentre review of practice. Thorax 2014; 69: 959–961. [DOI] [PubMed] [Google Scholar]
- 35.Bakirci T, Sasak G, Ozturk S, et al. Pleural effusion in long-term hemodialysis patients. Transplant Proc 2007; 39: 889–891. [DOI] [PubMed] [Google Scholar]
- 36.Ray S, Mukherjee S, Ganguly J, et al. A cross-sectional prospective study of pleural effusion among cases of chronic kidney disease. Indian J Chest Dis Allied Sci 2013; 55: 209–213. [PubMed] [Google Scholar]
- 37.Edwards SR, Unger AM. Acute hydrothorax – a new complication of peritoneal dialysis. JAMA 1967; 199: 853–855. [PubMed] [Google Scholar]
- 38.Nomoto Y, Suga T, Nakajima K, et al. Acute hydrothorax in continuous ambulatory peritoneal dialysis – a collaborative study of 161 centers. Am J Nephrol 1989; 9: 363–367. [DOI] [PubMed] [Google Scholar]
- 39.Johnston RF, Loo RV. Hepatic hydrothorax; studies to determine the source of the fluid and report of thirteen cases. Ann Intern Med 1964; 61: 385–401. [DOI] [PubMed] [Google Scholar]
- 40.Currie GP, Watt SJ, Maskell NA. An overview of how asbestos exposure affects the lung. BMJ 2009; 339: b3209. [DOI] [PubMed] [Google Scholar]
- 41.Cugell DW, Kamp DW. Asbestos and the pleura: a review. Chest 2004; 125: 1103–1117. [DOI] [PubMed] [Google Scholar]
- 42.Maldonado F, Tazelaar HD, Wang CW, et al. Yellow nail syndrome: analysis of 41 consecutive patients. Chest 2008; 134: 375–381. [DOI] [PubMed] [Google Scholar]
- 43.Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7: 198–201. [DOI] [PubMed] [Google Scholar]
- 44.Bynum LJ, Wilson JE III. Radiographic features of pleural effusions in pulmonary embolism. Am Rev Respir Dis 1978; 117: 829–834. [DOI] [PubMed] [Google Scholar]
- 45.Porcel JM, Madronero AB, Pardina M, et al. Analysis of pleural effusions in acute pulmonary embolism: radiological and pleural fluid data from 230 patients. Respirology 2007; 12: 234–239. [DOI] [PubMed] [Google Scholar]
- 46.Jurik AG, Davidsen D, Graudal H. Prevalence of pulmonary involvement in rheumatoid arthritis and its relationship to some characteristics of the patients. A radiological and clinical study. Scand J Rheumatol 1982; 11: 217–224. [DOI] [PubMed] [Google Scholar]
- 47.Balbir-Gurman A, Yigla M, Nahir AM, et al. Rheumatoid pleural effusion. Semin Arthritis Rheum 2006; 35: 368–378. [DOI] [PubMed] [Google Scholar]
- 48.Wang DY, Yang PC, Yu WL, et al. Serial antinuclear antibodies titre in pleural and pericardial fluid. Eur Respir J 2000; 15: 1106–1110. [DOI] [PubMed] [Google Scholar]
- 49.Doerr CH, Allen MS, Nichols FC III, et al. Etiology of chylothorax in 203 patients. Mayo Clin Proc 2005; 80: 867–870. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Zamalloa A, Ruiz-Irastorza G, Aguayo FJ, et al. Pseudochylothorax. Report of 2 cases and review of the literature. Medicine (Baltimore) 1999; 78: 200–207. [DOI] [PubMed] [Google Scholar]
- 51.Moore PJ, Thomas PA. The trapped lung with chronic pleural space, a cause of recurring pleural effusion. Mil Med 1967; 132: 998–1002. [PubMed] [Google Scholar]
- 52.Huggins JT, Sahn SA, Heidecker J, et al. Characteristics of trapped lung: pleural fluid analysis, manometry, and air-contrast chest CT. Chest 2007; 131: 206–213. [DOI] [PubMed] [Google Scholar]
- 53.Huggins JT, Sahn SA. Drug-induced pleural disease. Clin Chest Med 2004; 25: 141–153. [DOI] [PubMed] [Google Scholar]
- 54.Antony VB. Drug-induced pleural disease. Clin Chest Med 1998; 19: 331–340. [DOI] [PubMed] [Google Scholar]
- 55.Agusti AG, Cardus J, Roca J, et al. Ventilation–perfusion mismatch in patients with pleural effusion: effects of thoracentesis. Am J Respir Crit Care Med 1997; 156: 1205–1209. [DOI] [PubMed] [Google Scholar]
- 56.Doelken P, Huggins JT, Pastis NJ, et al. Pleural manometry: technique and clinical implications. Chest 2004; 126: 1764–1769. [DOI] [PubMed] [Google Scholar]
- 57.US Food and Drug Administration. 510(K) Premarket Notification. K971753. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?id=k971753 Date last updated: April 11, 2016. Date last accessed: March 9, 2016. [Google Scholar]
- 58.US Food and Drug Administration. 510(K) Premarket Notification. K121849. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?id=k121849 Date last updated: April 11, 2016. Date last accessed: March 9, 2016. [Google Scholar]
- 59.Olden AM, Holloway R. Treatment of malignant pleural effusion: PleuRx catheter or talc pleurodesis? A cost-effectiveness analysis. J Palliat Med 2010; 13: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puri V, Pyrdeck TL, Crabtree TD, et al. Treatment of malignant pleural effusion: a cost-effectiveness analysis. Ann Thorac Surg 2012; 94: 374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bintcliffe O, Arnold D, Maskell N. Indwelling pleural catheters for benign pleural effusions. Curr Respir Care Rep 2014; 3: 61–70. [Google Scholar]
- 62.Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet 2007; 369: 1535–1539. [DOI] [PubMed] [Google Scholar]
- 63.Maskell NA, Lee YC, Gleeson FV, et al. Randomized trials describing lung inflammation after pleurodesis with talc of varying particle size. Am J Respir Crit Care Med 2004; 170: 377–382. [DOI] [PubMed] [Google Scholar]
- 64.Steger V, Mika U, Toomes H, et al. Who gains most? A 10-year experience with 611 thoracoscopic talc pleurodeses. Ann Thorac Surg 2007; 83: 1940–1945. [DOI] [PubMed] [Google Scholar]
- 65.Glazer M, Berkman N, Lafair JS, et al. Successful talc slurry pleurodesis in patients with nonmalignant pleural effusion. Chest 2000; 117: 1404–1409. [DOI] [PubMed] [Google Scholar]
- 66.Sudduth CD, Sahn SA. Pleurodesis for nonmalignant pleural effusions. Recommendations. Chest 1992; 102: 1855–1860. [DOI] [PubMed] [Google Scholar]
- 67.Lee WJ, Kim HJ, Park JH, et al. Chemical pleurodesis for the management of refractory hepatic hydrothorax in patients with decompensated liver cirrhosis. Korean J Hepatol 2011; 17: 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milanez de Campos JR, Filho LO, de Campos Werebe E, et al. Thoracoscopy and talc poudrage in the management of hepatic hydrothorax. Chest 2000; 118: 13–17. [DOI] [PubMed] [Google Scholar]
- 69.Artemiou O, Marta GM, Klepetko W, et al. Pleurovenous shunting in the treatment of nonmalignant pleural effusion. Ann Thorac Surg 2003; 76: 231–233. [DOI] [PubMed] [Google Scholar]
- 70.Little AG, Kadowaki MH, Ferguson MK, et al. Pleuro-peritoneal shunting. Alternative therapy for pleural effusions. Ann Surg 1988; 208: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arai HI, Nishii K, Oyama T, et al. A pleuroperitoneal shunt for interactive pleural effusions with yellow nail syndome. J Med Cases 2011; 2: 115–120. [Google Scholar]
- 72.Tsang V, Fernando HC, Goldstraw P. Pleuroperitoneal shunt for recurrent malignant pleural effusions. Thorax 1990; 45: 369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]