ABSTRACT
First variants of the Klebsiella pneumoniae carbapenemase (KPC), KPC-2 and KPC-3, have encountered a worldwide success, particularly in K. pneumoniae isolates. These beta-lactamases conferred resistance to most beta-lactams including carbapenems but remained susceptible to new beta-lactam/beta-lactamase inhibitors, such as ceftazidime-avibactam. After the marketing of ceftazidime-avibactam, numerous variants of KPC resistant to this association have been described among isolates recovered from clinical samples or derived from experimental studies. In KPC variants resistant to ceftazidime-avibactam, point mutations, insertions and/or deletions have been described in various hot spots. Deciphering the impact of these mutations is crucial, not only from a therapeutic point of view, but also to follow the evolution in time and space of KPC variants resistant to ceftazidime-avibactam. In this review, we describe the mutational landscape of the KPC beta-lactamase toward ceftazidime-avibactam resistance based on a multidisciplinary approach including epidemiology, microbiology, enzymology, and thermodynamics. We show that resistance is associated with three hot spots, with a high representation of insertions and deletions compared with other class A beta-lactamases. Moreover, extension of resistance to ceftazidime-avibactam is associated with a trade-off in the resistance to other beta-lactams and a decrease in enzyme stability. Nevertheless, the high natural stability of KPC could underlay the propensity of this enzyme to acquire in vivo mutations conferring resistance to ceftazidime-avibactam (CAZavi), particularly via insertions and deletions.
KEYWORDS: KPC, ceftazidime-avibactam, epidemiology, stability, spectrum, trade-off, insertions, deletions, omega loop, KPC beta-lactamase, KPC-2, KPC-3, ceftazidime-avibactam resistance
INTRODUCTION
Carbapenem resistance in Enterobacterales is a major health issue as it complicates the therapeutic management of patients, and increases the morbidity and mortality in case of infection (1–7). A frequent mechanism of resistance relies on the production of carbapenemase (8–11).
The most prevalent carbapenemases are KPC, OXA-48, NDM and VIM-types with large disparities between countries and regions (8–12). Klebsiella pneumoniae carbapenemase (KPC) belongs to family of class-A serine beta-lactamases (12–14) and was found for the first time in 1996 in the United States (15). It is now a common carbapenem resistance mechanism among Enterobacterales in many countries including India, Mediterranean and European countries (5, 10–12, 16).
Historically, the KPC carbapenemases demonstrate a broad substrate profile, including penicillins, cephalosporins, aztreonam, carbapenems and are resistant to most standard beta-lactamase inhibitors (clavulanic acid, tazobactam, sulbactam). Recently, new beta-lactamase inhibitors have been developed with an activity on KPC beta-lactamase such as avibactam, and more recently vaborbactam and relebactam (17–24). The combination ceftazidime-avibactam (CAZavi) has been approved in 2015 in the USA and in 2016 in Europe. CAZavi demonstrates excellent in vitro activity against KPC-producing Enterobacterales and is associated with a decreased mortality rate in treated patient (22, 25, 26). However, since commercialization, many alerts have been raised on the emergence of resistant KPC-mutants after therapy with CAZavi (27, 28), showing a high evolutionary potential of this enzyme. To date (July 2022), 65 KPC variants resistant to CAZavi have been described. These variants harbor mutations (insertions, deletions and/or point mutation) and many of them differ in resistance phenotype, including in their level of resistance to carbapenems. Here, we describe the mutational landscape of the KPC beta-lactamase toward ceftazidime-avibactam resistance in clinical isolates including epidemiology, microbiology, enzymology, and thermodynamics data.
EPIDEMIOLOGY OF KPC-PRODUCING ENTEROBACTERALES
After the first identification of KPC-2 in 1996 in a Klebsiella pneumoniae isolate in North Carolina (15), KPC-2 producing Enterobacterales rapidly spread in hospitals on the US East coast (29). The KPC-3 variant, differing by the H274Y mutation, was then described in K. pneumoniae (30) during an outbreak in a New York hospital in 2000. Since that time, the incidence of KPC-producing K. pneumoniae strains has been increasing (11, 15, 29) and KPC beta-lactamase is now one of the most common carbapenemase detected globally and reaches up to several percent among K. pneumoniae isolates in some European countries (5, 11, 12, 16, 23, 31, 32).
Among countries monitored by the ECDC (European Centre for Disease Prevention and Control), most have reported an increase in the incidence of carbapenemase-producing Enterobacterales (CPE) between 2016 and 2020 (33).
In the European Union (EU) or European Economic Area (EEA), resistance to carbapenems in Escherichia coli isolates and K. pneumoniae isolates represented 0.2% and 6.8%, respectively, of the total number of invasive isolates in 2015. It has remained stable for E. coli but increased to 10.0%, in 2020 for K. pneumoniae isolates (33). The endemic stage has even been declared successively in Israel, Greece and Italy (34, 35). Between 2005 and 2014 in Greece, among the 3,449 K. pneumoniae isolates collected by the University Hospital of Patras, 48% were carbapenemase-producing, including 80% of KPC-type (36). In a study conducted between 2014 and 2016 in 15 Greek hospitals, KPC-type enzymes accounted for 66.5% of the 394 carbapenemase-producing K. pneumoniae (34). Conversely, if the number of episodes involving CPE isolates rose in France during these last 2 decades, KPC-producing Enterobacterales represented only 2.9% of CPE sent to the National Reference Center in France in 2020 (37).
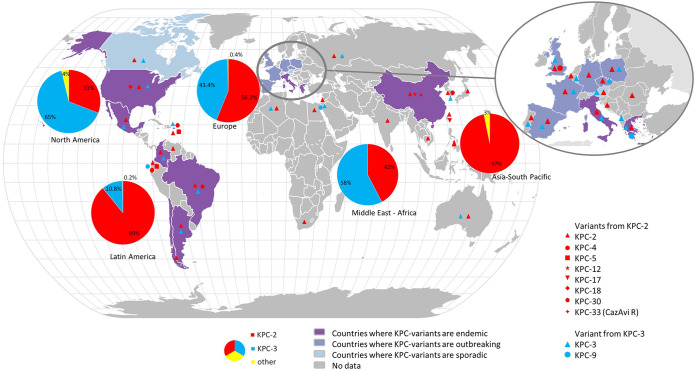
A worldwide surveillance study that collected 38,266 Gram-negative pathogens from 2012 to 2014 found 586 isolates (1.2%) of KPC-type carbapenemase (38). KPC-2 was the most prevalent (408, 69.6%) followed by KPC-3 (173, 29.5%), KPC-9, KPC-12 and KPC-18 which were respectively found in 2, 1 and 2 patients. A different distribution was observed in Italy, Israel or the United States in which KPC-3 variants are predominant. Interestingly, the strains isolated in Israel in 2004 were genetically close to that identified in the United States in the 2000s (30, 39), suggesting worldwide diffusion by travelers (34, 40). A second worldwide surveillance study included 81,781 enterobacterial isolates from 39 countries in 5 geographic regions between 2012 and 2017. The global prevalence of meropenem non-susceptible isolates was 2.8% with geographical disparities ranging from 1.2% in North America to 4.3% in Latin America. Among these non-susceptible isolates, KPC-2 and KPC-3 were found respectively in 27.3% and 21% of isolates in Europe for a total of 1284 non-susceptible isolates. In Latin America, KPC-2 and KPC-3 were found in 79.6% and 9.5% of the isolates (for a total of 525 isolates), in Asia/Pacific, KPC-2 was found in 25.5% of the isolates while KPC-3 was not found (231 isolates in total), in Middle East Africa, KPC-2 and KPC-3 represented 8.7% and 12% respectively (total of 126 isolates) and finally, in North America KPC-2 and KPC-3 were found in 61 and 28.7% of meropenem non-susceptible isolates respectively (total of 87 isolates). Other variants have been described from time to time such as KPC-9, -12, -17, -18, -29 and –30 (41) (Fig. 1, Table S1). Unsurprisingly, the variants derived from KPC-2 and KPC-3 have a geographic distribution that overlaps with that of their ancestor.
FIG 1.
Geographical distribution of KPC-2 and KPC-3 and their most frequent circulating variants. The data used to generate this figure are listed in supplementary table 1.
Genetic background analyses revealed the worldwide spread of KPC was related to the release of some major clones of K. pneumoniae initially belonging to the ST258 (42) and more recently to others like ST512 (43–45). Although KPC β-lactamases are more frequently associated with K. pneumoniae, they are also identified in many other species including E. coli, Citrobacter sp., Enterobacter sp., Serratia marcescens, Proteus mirabilis, Morganella morganii, as well as in non-fermentative Gram-negative bacilli strains such as Pseudomonas aeruginosa and Acinetobacter baumannii (16, 46). KPC-type carbapenemase gene is frequently located on self-conjugative plasmids, variable in size and number, and often associated with the Tn4401 transposon (type 3) (12, 47), encouraging its worldwide diffusion between species.
In addition to hospital and community dissemination in humans, KPC variants have been found in domestic, farmed, and wild animals as shown in the review of Köck et al. published in 2018 which compiles the data of nearly 70 articles (48). In particular, the KPC genes were found in birds (49, 50) and in chicken meat (51). In addition, other works showed an environmental diffusion in different sources and, in particular, aquatic (48, 52). For example, a multi-resistant enterobacteria harboring blaKPC was found in a mollusk sold on a market in Tunisia (53) or in wastewater in Austria and Brazil (54). The human origin of these isolates cannot be ruled out. However, this suggests a global diffusion of KPC genes in different environments.
STRUCTURE OF KPC CARBAPENEMASE
KPC enzymes are proteins of 293 amino acids in average, with a molecular weight of c.a. 32 kDa. Fifty-eight KPC-2 structures, or variants, are currently available in the Protein Data Bank (https://www.rcsb.org/) (55). They are composed of two subdomains and the overall folding is similar to that observed in other class A beta-lactamases (13, 56). The first subdomain is composed of 8 alpha helixes, and the second is composed of 5 antiparallel beta sheets flanked by 3 alpha helixes (56, 57). These two domains share electrostatic interactions, and hydrogen bridges stabilize the structure. The active site, located in a pocket at the interface, contains the catalytic S70 residue and the deacylation water, that is activated when interacting with E166, N170 and S70 (56).
It is worth reminding that the hydrolysis of the amide bond of β-lactam by beta-lactamase involves an acyl-enzyme covalent intermediate close to the O atom of the invariant serine 70 residue which plays the role of nucleophile. Then, an activated water molecule attacks the covalent complex, resulting in subsequent hydrolysis of the bond between β-lactam carbonyl and serine oxygen. This leads to the regeneration of the active enzyme and the release of the inactive antibiotic β-lactam (58).
In KPC, as in the other Ambler Class-A enzymes, very conserved regions or motifs that contribute to the catalytic function of the enzyme are identified (13, 14, 59) (Fig. 2).
FIG 2.
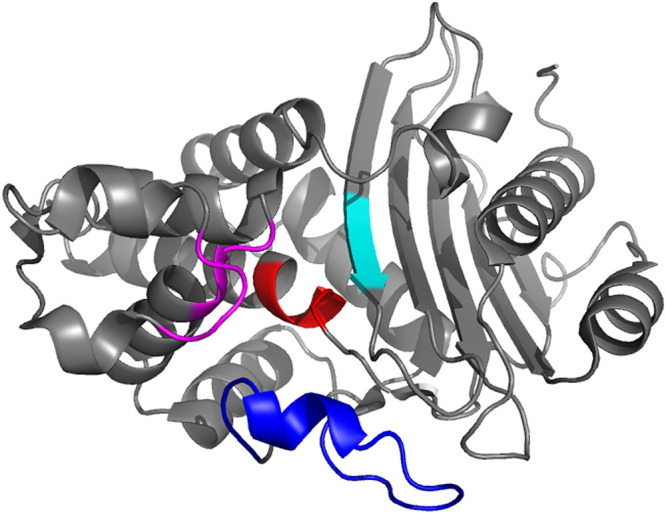
Representative view of the overall KPC-2 fold (PDB 5UL8), showing positions of key active-site residues and loops. The S70XXK73 motif is colored in red, residues 104–106 and S130D131N132 loop in magenta, the K234T235G236 pattern in cyan, and the Ω-loop in blue.
(i) The first motif is the S70XXK73 sequence containing the main catalytic residue Ser70. Lys73 which is strictly conserved and connected to the Ser70 by a hydrogen bond is of importance for the enzyme’s function. The active site is delimited by three residues (residues 104, 105 and 240) (60). The W105 residue is important for the ligand recognition (61) and could favor interactions with substrates such as carbapenems (56).
(ii) The second conserved pattern is the S130D131N132 loop, where Ser130 is probably involved in proton transfer from Ser70 to the β-lactam nucleus during the acylation step.
(iii) The third motif, the K234T235G236 pattern, is important in the structure since it is located at the right hand-side of the active site. Crystallographic data indicate that Ser130 and Lys234 are connected by a hydrogen bond, which would serve as a connection between the two domains of the protein and help stabilize the active site (62).
(iv) The Ω-loop is a pattern found in all class A beta-lactamases, that contains 16 residues (from Arg164 to Asp179), including the E166-X-G168L169N170 sequence, in which the Glu166 and Asn170 residues are important for positioning the water molecule (57, 63).
EMERGENCE OF KPC VARIANTS RESISTANT TO CAZAVI
The first CAZavi-resistant strain was a K. pneumoniae described in 2015, the year CAZavi was marketed. Surprisingly, this strain was isolated from a patient who had not received this drug. Resistance was probably due to efflux (64). Since then, most new KPC-type clinical variants described in the databases are associated with resistance to CAZavi. Although these resistances remain at low rates in surveillance studies (33, 38, 41, 65), they might increase in the future with the use of CAZavi or other new beta-lactamase inhibitors.
The mechanisms of resistance to CAZavi in KPC-producing strains could be due to co-production of less sensitive beta-lactamases (e.g., MBLs), to changes in membrane permeability including loss or mutations in porins, or to efflux pumps (65). Nevertheless, the most frequent mechanism remains as mutations in KPC-encoding genes.
Di Bella et al. reviewed the CAZavi resistance from a clinical point of view in 42 patients from 23 clinical studies or case reports (66). They showed that most isolates (55/57) were K. pneumoniae which were mainly identified in the USA, Italy and Greece, countries where prevalence of KPC-producing K. pneumoniae is high. The most frequent clinical variants were KPC-31 and KPC-33 involving the D179Y +/- H274Y mutation. Other infrequent variants such as KPC-8 (V240G-H274Y), KPC-23 (V240A-H274Y) or KPC-40 (T237S-H274Y) have also been described (66). In a descriptive study, Venditti et al. found 26/31 patients with a mutated KPC and reported a majority of KPC-31 and KPC-29 alleles (67).
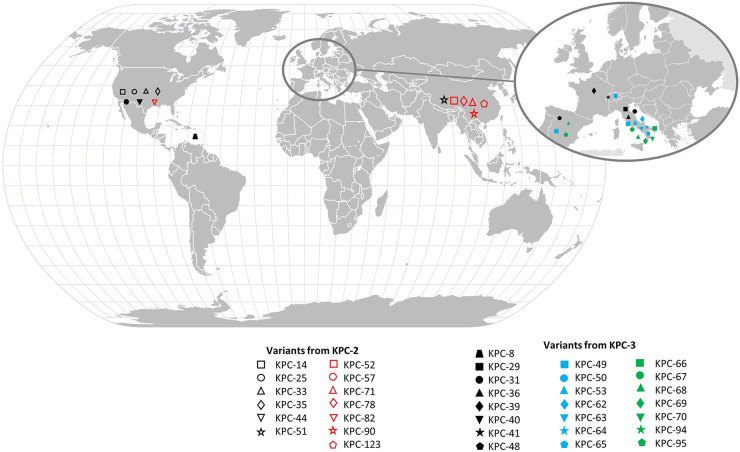
The different KPC variants conferring resistance to CAZavi and for which the literature data specify their geographical origin are listed in Fig. 3 and Table S2.
FIG 3.
Geographical distribution of CAZAVI-resistant KPC-2 and KPC-3 alleles. The data used to generate this figure are listed in supplementary table 2.
STRUCTURAL INSIGHT INTO THE FUNCTIONAL EFFECTS OF MUTATIONS CONFERRING RESISTANCE TO CAZAVI
Below, focusing on the mutations associated with resistance to CAZavi, we give an evolutionary perspective integrating the concept of structure-activity relationship as well as the underlying molecular mechanisms.
Of the 126 clinical variants listed in the NCBI database (july 2022), 65 are described as resistant to CAZavi, represented in Table 1 and with references and accession numbers in Table S3. Of these, 36 are KPC-2 variants, and 29 are KPC-3 variants. It appears that 3 mutational hot spots are associated with resistance to CAZavi (Fig. 4 and Table 1): (i) the Ω-loop region (residues 164 to 179 bordering the lower part of the catalytic pocket), (ii) loop 237–243 (located between beta 3 and beta 4, close to the conserved KTG motif and delimiting the right side of the active site), and (iii) loop 266–275 (located at a distance from the active site between beta 5 and alpha 11 helix). It should be noted that some mutations, particularly insertions, occur in the vicinity of these hot spots with a probable direct impact in the loop itself.
TABLE 1.
Alleles of KPC involved in resistance to ceftazidime-avibactam
| KPC-varianta | Position | Omega loop | Loop 237-243 | Loop 266-275 | Other mutation |
|---|---|---|---|---|---|
| KPC-117* | 2 | Ins_2_RCP/L3I/Y4F | |||
| KPC-36 | 163 | D163E | |||
| KPC-49 | 164 | R164S | |||
| KPC-98* | 164 | R164H | |||
| KPC-53 | 165 | ins_165_EL | |||
| KPC-40 | 165 | ins_165_EL | T243S | ||
| KPC-25 | 165 | ins_165_EL | |||
| KPC-112* | 165 | ins_165_EL | del_242-243_GT | ||
| KPC-69 | 165 | ins_165_GL | |||
| KPC-113* | 166 | Ins_166_G | |||
| KPC-73* | 168 | del_168-169_EL | ins_269_KDDKHS | ||
| KPC-66 | 168 | del_168-169_EL | |||
| KPC-94 | 169 | L169H/Del_170_N | |||
| KPC-35 | 169 | L169P | |||
| KPC-46* | L169P | ||||
| KPC-48 | 169 | L169P/A172T | |||
| KPC-62 | 169 | L169Q | |||
| KPC-115* | 169 | del_169-170_LN/S171P | |||
| KPC-72* | 172 | A172D | |||
| KPC-39 | 172 | A172T | |||
| KPC-95 | 172 | A172T/D179Y | |||
| KPC-85* | 172 | A172V | |||
| KPC-70 | 176 | D176Y | |||
| KPC-88* | 176 | D176Y | |||
| KPC-78* | 179 | D179A | ins_265_RAPNKDDN | ||
| KPC-86* | 179 | D179G | |||
| KPC-51 | 179 | D179N | Y241H | H274N | |
| KPC-57 | 179 | D179V | |||
| KPC-76* | 179 | D179Y | ins_262_VYTRAPN | ||
| KPC-32* | 179 | D179Y | T243M | ||
| KPC-31 | 179 | D179Y | |||
| KPC-33 | 179 | D179Y | |||
| KPC-90* | 179 | Ins_179_TY | |||
| KPC-65 | 179 | ins_179_TY | |||
| KPC-52 | 179 | D179Y | ins_262_V | ||
| KPC-68 | 180 | ins_180_SS | |||
| KPC-114* | 180 | ins_180_SS | |||
| KPC-71* | 180 | ins_180_S | |||
| KPC-64 | 180 | ins_180_S | T243A | Y264H | |
| KPC-123 | 180 | ins_180_YT | ins_271_DDKHSEA | ||
| KPC-107* | 180 | Ins_180_SSPRAVTESLAQKLTLGSALAAPQRQQFV | |||
| KPC-108* | 181 | Ins_181_SPRAVTENTS | Ins_262_VYTRAPNKDDKHSEA | ||
| KPC-104* | 181 | S181Y/Ins182_TS | Ins_271_DDKHSEA | ||
| KPC-106* | 181 | S181Y/Ins182_TS | Ins_275_SEAV | ||
| KPC-74* | 239 | del_239-240_GV | |||
| KPC-23 | 240 | V240A | |||
| KPC-8 | 240 | V240G | |||
| KPC-63 | 241 | Y241S | |||
| KPC-96* | 241 | Y241N | |||
| KPC-28 | 242 | del_242-243_GT | |||
| KPC-14 | 242 | del_242-243_GT | |||
| KPC-87* | 242 | del_242_G/ T243A | |||
| KPC-84* | 243 | T243P | |||
| KPC-44 | 261 | ins_261_AVYTRAPNKDDKHSE | |||
| KPC-79* | 262 | ins_262_VYTRAPN | |||
| KPC-105* | 263 | Ins_262_VYTRAPNKDDKHSEA | |||
| KPC-58* | 265 | ins_265_RAPNKDDN | |||
| KPC-41 | 267 | ins_267_PNK | |||
| KPC-80* | 267 | ins_267_PNK | |||
| KPC-29 | 269 | ins_269_KDD | |||
| KPC-67 | 269 | ins_269_KDDKDD | |||
| KPC-103* | 270 | Ins_270_KDDKHSEAVIAA | |||
| KPC-50 | 275 | ins_275_EAV | |||
| KPC-82 | 275 | ins_275_SD | |||
| KPC-97* | 276 | Ins_276_VNSEA |
Variants indicated with a star correspond to variants unpublished, but indicated as resistant to ceftazidime-avibactam in the NCBI Nucleotide Database. Accession Number and References are available in the Supplementary Table 3.
FIG 4.
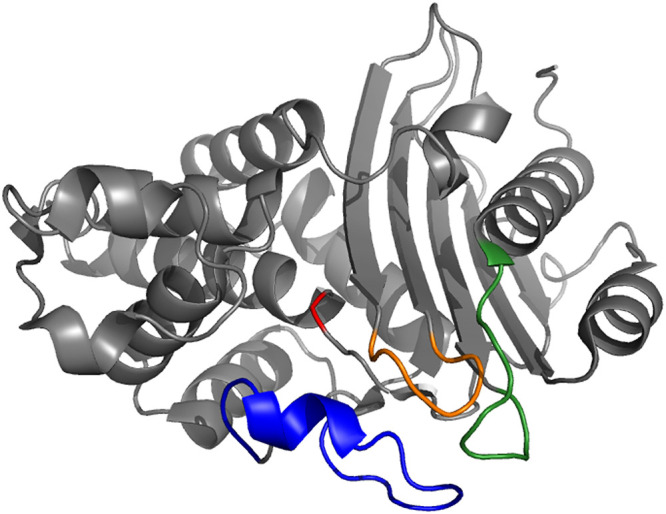
Representative view of the overall KPC-2 fold (PDB 5UL8), showing the 3 hot spots involved in ceftazidime-avibactam resistance: the omega loop is colored in blue, the 238–243 loop in orange and the 267–275 loop in green. For indication, S70 is colored in red.
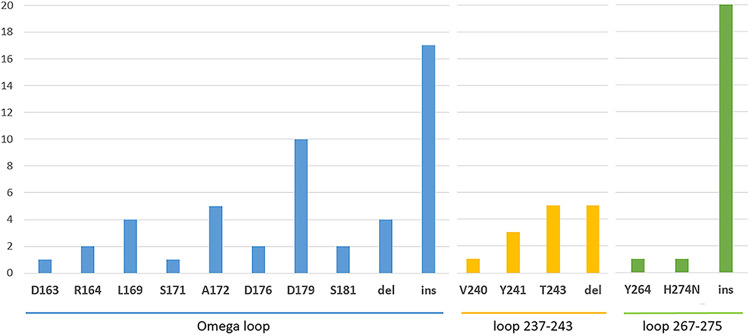
Altogether, 43, 14, and 20 variants resistant to CAZavi harbor mutations in the Ω-loop region, in the loop 237–243 and in the loop 266–275 region, respectively. Of note, some mutants can have multiple mutations in these different regions. A significant proportion of KPC clinical variants resistant to CAZavi (41/65, 63%) harbor insertions and/or deletions (indels), associated or not with point mutations. The insertions correspond mostly to sequence duplications. Seventeen insertions and four deletions are present in the Ω-loop regions; Five deletions in the loop 237–243, and 20 insertions ranging in size from 1 to 15 amino acids in the loop 266–275 (Fig. 5).
FIG 5.
Distribution of point mutations and indel in the different hot spots conferring resistance to CAZavi in KPC variants.
Overall, the Ω-loop appears to be permissive to insertions and deletions, the loop 237–243 only to deletions, and the loop 266–275 only to insertions suggesting that each structure exhibits a different mutational tolerance. We will now describe the mutational tolerance of each of these 3 structures in KPC beta-lactamase and identify the mechanisms related to the structure-activity relationship.
Mutations located in the Ω-loop.
The Ω-loop (residues 164–179) surrounds the active site of KPC-2, adjacent to the catalytic S70. It is a hot spot for substitutions that widen the substrate spectrum of many class A enzymes (TEM-1, SHV-1, etc) toward ceftazidime (9, 68, 69). It contains Glu166 and Asn170, 2 essential residues required for activating a water molecule for the β-lactam deacylation (70). Structurally, the Ω-loop is largely stabilized by a saline bridge between the R164 and the D179 residue. This Ω-loop is fundamental for maintaining the structure of the protein and interacts with many residues outside this loop. Particularly, D179 connects to residues outside the Ω-loop like Pro67, Leu68 et Arg161 by hydrogen bonds (71). Thus, point mutations can have repercussions on the Ω-loop but also outside. So, due to their central role in the interactions between KPC-2 residues, it is not surprising to frequently find variants with point mutations at position 164 (n = 2) and 179 (n = 10) in clinical alleles resistant to CAZavi.
Position 163, directly adjacent to the Ω-loop, is involved in point mutations like the D163E substitution associated with H274Y in KPC-36 that confers resistance to CAZavi. This position is also found in other class A beta-lactamases as associated with an extension of resistance to ceftazidime in TEM-1 (68, 72). The underlying mechanism is probably related to the contiguity with position 164 which plays a major role in the stability of the loop.
The Arg164 residue is important for maintaining the Ω-loop stability (69) and notably the salt bridge’s integrity with Asp179 that insures Glu-166’s position, important for the deacylation step (72). A point mutation in this site like R164S, found in KPC-49 (also associated with H274Y), leads to a disturbance on this conserved bridge and show increased CZA MICs (71). Levitt et al. (73) built 19 mutants with a single substitution at Ambler position R164, and showed 58% of resistance to ceftazidime in these mutants. They showed slow hydrolysis compared to acylation (and therefore increased accumulation of acyl-enzyme), resulting in a form of ceftazidime “covalent trapping” by R164S (73). Winkler et al. showed that avibactam was equally efficient on wild type KPC-2, and on R164A and D179N variants (74). Hence, the CAZavi resistance could result from an increased and rapid binding of ceftazidime while avibactam cannot reach the active site (74). The consequence of these structural modifications is a widening of the active site, which increases resistance to large beta-lactams, such as ceftazidime (72). Interestingly, point mutations at that position also confers a broadening of the spectrum toward ceftazidime in TEM-1, SHV-1 and CTX-M type enzymes (72, 75), underlining the important role of this site in the function of class A beta-lactamases toward ceftazidime.
Six KPC clinical alleles have insertion at positions 165–166 (KPC-25, -40, -53, -69, -112 and -113) either alone or in combination with a point mutation (H274Y or T234S) or a deletion in the loop 237–243 (del_242-243_GT) (45, 76). The same type of insertions (165-YYG-167) have been found in TEM-1 and studied by Petrosino et al. (69). They reported a 100-fold ceftazidime hydrolysis increase compared to the wild type. This is probably due to the change in the size of the Ω-loop, creating instabilities at the structural level, and subsequently modifying the size of the active site, eventually affecting the hydrolytic capacities. However, this mechanism remains to be clearly studied.
The deacylation water is primed by interacting with E166, N170 and S70 (56), therefore placing the Glu166 site as of paramount importance in the catalytic mechanism (77). This explains why no point mutation is found at this site and why the E166 is a conserved residue in class A beta-lactamases (78).
At position 169, a deletion of the E168L169 motif is identified in KPC-66 and KPC-73 variants and is also described in laboratory isolates (67, 79, 80). In addition, the L169P and L169Q point mutations are associated with reduced susceptibility to CAZavi in 4 clinical variants (KPC-35, -46, -48 and -62) (67, 79–81).
There are no clinical alleles with a point mutation at the N170 position. However, Gottig et al. (82) showed that the N170D point mutation led to an increase in the MIC of CAZavi from 0.5 to 32 mg/L. As previously seen, the N170 site plays a role in the active site structure and establishes hydrogen bonds between E166 and water molecules (78). It is a conserved residue in class A beta-lactamases suggesting that it has a primary role. It’s thus surprising to see recently described clinical alleles KPC-94 and KPC-115 with deletions that include N170 (83). However, avibactam makes van der Waals interactions with N170 and a hydrogen bond with the diacylation water that is held in place by E166 and N170. The loss of these interactions with avibactam probably participate to the resistance to ceftazidime-avibactam (84, 85).
At position 172, 5 variants are found and thought to be resistant to CAZavi; A172T (KPC-39), A172D (KPC-72), A172V (KPC-85), A172T-D179Y (KPC-95) and A172T-L169P (KPC-48) (83, 86).
At position 176, the D176Y point mutation, alone or in combination with H274Y, is found in the KPC-70 and KPC-88 (45). Few details are available on this site but its impact on resistance to CAZavi was also shown in laboratory isolates (82) and is possibly due to disorder in the Ω-loop.
Position 179 is one of the most common position reported in CZA resistant isolates (27, 80, 87–90) with a variety of substitutions in 10 different clinical isolates (D179Y, D179N, D179A, D179G and D179V found respectively in KPC-31/32/33/52/76/95, KPC-51, KPC-78, KPC-86, and KPC-57) and an insertion Ins179TY found in two other isolates (KPC-65 and KPC-90) (67), usually associated with other mutations (82, 91–93). Point mutations have also been characterized by Barnes et al. in laboratory isolates confirming their implication in CAZavi resistance (71, 82).
In particular, the D179N mutation confers resistance to CAZavi while maintaining a hydrolytic profile close to KPC-2 (85). The D179Y mutation that is the most frequently reported, increases CAZavi MIC while reducing meropenem MIC (27, 76, 79, 88, 94, 95). Of note, Shields et al. reported a possibility of reversion to the ancestral KPC-3 allele, thus restoring susceptibility to the strain (94).
At the mechanistic level, different hypotheses explain resistance to CAZavi. The point mutation in position 179 leads to a break of the salt bridge with the R164 position, which destabilizes the structure of the loop, but also prevents hydrogen bonds between D179 and other residues distant from the Ω-loop, such as Pro67, Leu68 and Arg161 (71).
At the enzymological level, the impact of D179Y and R164 point mutations are similar, associated with a displacement of the E166 and N170 residues. Changes in the Ω-loop allow the ceftazidime a better access to the active site, associated with a covalent trapping of the ceftazidime antibiotic in the active site (71, 73, 85). Regarding the effect of avibactam, two mechanisms seem to contribute to a decrease in binding: 1/The covalent trapping of ceftazidime prevents avibactam from binding to and inhibiting the enzyme (71, 74); 2/the D179Y has a significant effect on the efficiency of avibactam acylation by the enzyme (70,000-fold decrease in the inactivation constant k2/K value) (91). This is probably due to the destabilization of the Ω-loop that prevents avibactam to create hydrogen bonds with the diacylation water, which is maintained by residues E166 and N170 (85). A recent molecular dynamic simulation and docking analysis performed by Taracila et al. also showed that the omega loop becomes larger and more flexible in the D179Y variants (96). This apparent disorder is made possible by a decrease in stability of the protein from 54–55°C to 47.5–51°C in the presence of the D179Y point mutation (96).
At position 180, duplication of a serine alone (KPC-71) or associated with other point mutations (KPC-64) have also been evidenced in vitro (67, 97). The insertion of two amino acids (SS or YT) is also described by the same author in the KPC-68 variant, in association with the H274Y point mutation and more recently in KPC-114 or KPC-123 (45, 98). A large insertion of 29 amino acids is also reported in KPC-107. These insertions, although located at the end of the Ω-loop, can also modify its structure by increasing its size, widening the active site.
At positions 181 and 182, point mutations and/or insertions are described and are associated with insertions in loop 267–275 in KPC-104, -106, and -108.
Thus, the Ω-loop which contains numerous mutations conferring resistance to CAZavi is a major site in the KPC protein, and more generally in class A beta-lactamases. Indeed, the salt bridge located between positions 164 and 179 is essential to maintain the structure of this loop which also interacts with remote positions. Mutations occurring in this site may lead to further modifications in the overall structure of the protein. Consequences can be a better access of the ceftazidime to the active site (63) and a higher affinity for ceftazidime that comes with a concomitant decrease in both Kcat and catalytic efficiency (as shown in D179 variants) (96). This often leads to changes in susceptibility to other beta-lactams like carbapenems. Some mutations in this loop also decrease the binding of avibactam, and lead to a reduction in the rate of carbamylation leading to resistance to the CAZavi combination (58, 63).
Mutations located in the loop 237–243.
In total, mutations in this loop were described in 14 variants. Interestingly, these variants often have associated mutations in other areas of beta-lactamase and in particular the Ω-loop. Here are the important sites that may be involved in resistance to CAZavi.
Residue 237 is implicated in the carbapenemase activity in class A carbapenemases and especially KPC (99, 100) as it is part of the active site (56). The length of the active site’s pocket is crucial for the carbapenemase activity and the interactions between the E166 and N237 determine this distance (56). It allows a good position of the beta-lactam carbonyl for the beta-lactam bond to be hydrolyzed. Papp Wallace et al. showed in vitro an important role of T237 in resistance to inhibitors but the study was performed before the CAZavi commercialization (101), therefore, this combination was not evaluated. No mutations are described at this position in clinical alleles.
Positions 239–240. The residue 239 is close to the active site, explaining mutations could impact the enzyme’s activity (100). For the positions 239 and 240, the deletion of the pattern G239V240 confers resistance in KPC-74, and in isolates from experimental evolution (102). In addition, V240G and V240A point mutations are described in KPC-8 and KPC-23, respectively (87, 89, 94) and in laboratory mutants (103) as being associated with CAZavi resistance. V240G and V240A point mutations are described to enlarge the active site for wider substrates/ligands to bind. In Mehta et al. study, the V240G substitution strongly contributed to the hydrolysis of ceftazidime (103). Interestingly, KPC-8 (V240G-H274Y) showed resistance to almost all beta-lactam including ceftazidime and carbapenems (103). The combination of P104R:V240G mutations in KPC-4 has been shown to induce a 40-fold increase of ceftazidime Kcat/Km compared with KPC-2. High-resolution crystal structures inform us that after ceftazidime is bound, these mutations could lead to a reduction in flexibility or an increase in the stability of the Ω-loop. This could constrain E166 in an orientation compatible with effective deacylation which is not observed with KPC-2 (63).
At position 241, Y241H and Y241S point mutations are described as probably resistant to CAZavi (67, 93). Indeed, in KPC-51, Y241H is associated with D179Y and H274N which makes it difficult to interpret the impact of mutations independently. However, Gottig et al. showed Y241N-mutated isolate was resistant to CAZavi, suggesting a possible important role of this position (82), recently described in a clinical mutant (KPC-96).
At position 242, deletions in positions 242-GT-243 were found in KPC-14, -28 and -112. KPC-14 and KPC-28 were well studied by Oueslati et al. (104). Their molecular models showed that deletion did not alter the overall structure of the protein, but resulted in a shorter 237–243 loop, shifting A244 slightly. The new A244 position led to a clash between the A244 side chains and the residue in position 274. This can expand the active site and allows better access to the substrate and deeper positioning of the aminothiazole cycle of ceftazidime in the active site (104). These structural changes are likely due to the disruption of interactions with the Ω-loop; V240, Y241 and T243 interact with residues 170–174 of the Ω-loop (85).
Mutations at position 243 are found once alone in KPC-84 (T243P) and 3 times associated with point mutations or indels in the Ω-loop as in KPC-32 (T243M), KPC-40 (T243S), and KPC-64 (T243A) or the loop 237–243 as in KPC-87 (del_242_G/T243A).
The respective role of each point mutation in resistance to CAZavi is not clearly defined. For Shields et al., and Haidar et al., the T243M point mutation appears to potentiate resistance to CAZavi when associated with the D179Y point mutation (76, 87). Livermore et al. show that T243P, found in the KPC-84 allele, increases the MIC to CAZavi, which does not seem to be the case for the T243A mutation (76, 87, 97). Recently, Alsenani et al. described that T243 is making van der Waals interactions with the Ω-loop and therefore a mutation at this site can lead to enhance destabilization of the omega loop (85).
The loop 237–243 has essential residues for the enzyme activity and in particular, site 237 which is part of the active site of class A beta-lactamases.
Mutations located in the loop 267–275.
The loop 267–275 is a hot spot for mutations since it is the 2nd site with the most mutations with 20 clinical isolates having mutations associated with resistance to CAZavi. All insertions within this region result in a CAZavi resistance phenotype. The number of inserted amino acids is comprised between 1 and 15. Among the few described in the literature, the duplication of 15 amino acids in position 261 (Ins261_AVYTRAPNKDDKHSE) in KPC-44 (105) and of 3 amino acids in position 267 (Ins267_PNK) (KPC-41 and KPC-80), 269 (Ins269_KDD) (KPC-29) and 275 (Ins275_EAV) (KPC-50) were associated with an increased affinity of ceftazidime but a reduced hydrolysis, along with a reduced sensitivity to avibactam (45, 106, 107). This illustrates a phenomenon relatively like the one conferred by mutations in position D179 responsible for a trapping of ceftazidime with diminished hydrolytic capacities. A similar impact on the hydrolysis spectrum is observed whatever the precise location of these duplications in the loop (105–108).
Thus, these insertions within the loop 267–275 could modify the conformation and increase the accessibility to the substrate. They would therefore result in an increased affinity toward ceftazidime, and a decreased inhibitory activity of avibactam, explaining the phenotype of resistance to CAZavi. Insertions of up to 15 amino acids have been reported, which greatly expands the size of the loop. In contrast to the other loops, very few mutations have been described in the wild within loop 266–275 in the other class A beta-lactamases, suggesting a very specific mechanism to KPC (103). However, some variants with mutations in this region have been observed in isolates from in vitro laboratory selection (58, 109).
The main point mutation found at site 274 is H274Y. This substitution, associated with resistance to ceftazidime, is found in KPC-3 and illustrates a high evolutionary success since it is identified in 29/65 (44.6%) clinical variants resistant to CAZavi.
A hydrogen bond between the hydroxyl side chain of tyrosine from the H274Y and the amine functionality of the ceftazidime’s aminothiazole ring is suggested to be involved in increased catalytic efficiency (103). The consequence is a 9-fold increase in catalytic efficiency compared to KPC-2 against ceftazidime (30, 110).
Interactions between hot spots.
Galdadas et al. studied the loop dynamics in KPC-2 and TEM-1 beta-lactamases (58). For them, distal loops are involved in the structure of the active site, through their connections with other sites in the protein (57, 111, 112). In TEM-1 and KPC-2, several loops surround the active sites. The highly conserved Glu166 and Asn170 residues are not only essential for the Ω-loop’s structure (113) but they also influence the other loops conformation. Both V240 and T243 are making van der Waals interactions with the Ω-loop explaining why this loop can participate in destabilizing the Ω-loop (85).
Changes in the loop 267–275 are necessary for a correct repositioning of the loop 237–243 to avoid steric clashes with the ceftazidime aminothiazole ring. According to Tooke et al., this could be made possible through deletions or substitutions in the loop 237–243 (63). Notably, H274Y substitution, involved in increase in MIC to CAZ, can further reduce the energy load associated with the reorganization of the loop 237–243 (63, 103).
MUTATION TRADE-OFF IN THE RESISTANCE SPECTRUM
While biochemical data have shown that KPC-2 has good hydrolytic activity on penicillins, cephalosporins and carbapenems, it poorly hydrolyzes cephamycins and ceftazidime (15). The KPC-3 variant exhibited a ceftazidime hydrolysis capacity greater than KPC-2 and a similar hydrolysis rate for carbapenems (104). In Mehta et al., most variants (KPC-2 to -11) increased activity toward ceftazidime without compromising the hydrolysis of carbapenems or penicillins (103). However, these variants have not been described regarding resistance to CAZavi (103).
What emerges from most studies describing KPC, CAZavi-resistant variants is an evolutionary trade-off with a decreased resistance to other beta-lactams, such as carbapenems (13, 94, 104). This is not surprising, as the acquisition of a new function in beta-lactamases is often correlated with the loss of a natural function. This is the case for TEM-1, in which there is a compromise at the hydrolysis spectrum level explained from a structural point of view: some of these mutations confer an enlargement of the pocket of the active site, allowing the interaction of the active site with oxy-iminocephalosporins, larger molecules, to the detriment of interaction with penicillins. There is therefore a broadening of the hydrolysis spectrum of penicillins, their natural substrate, toward oxy-iminocephalosporins (114). Regarding KPC carbapenemase, the same compromise is observed.
It is interesting to note that the 3 key regions involved in CAZavi resistance are involved in this change in activity: Within the Ω-loop, substitutions in position 179 alter the hydrolysis of natural substrates such as ampicillin, aztreonam and meropenem. The same applies to substitutions in positions 163, 164, 169 and 172. The structural modification and disorganization of the saline bridge therefore has a major impact on the hydrolytic spectrum. As an example, a drastic reduction of MICs to carbapenems is observed in KPC-25 and KPC-40, related to the insertion of 2 amino acids, Ins165_EL (76, 115).
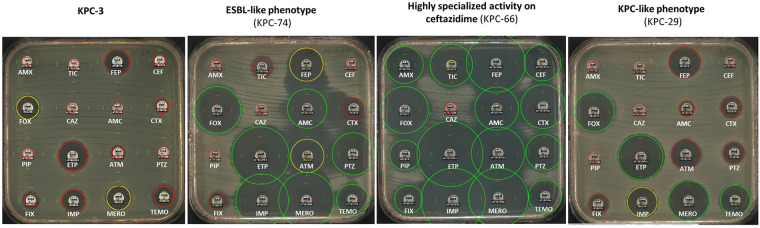
In a study carried out by Hobson et al. (102) on clinical and in vitro variants resistant to CAZavi, we showed that insertions in the Ω-loop resulted in a very different resistance profile (Fig. 6). We observed ESBL-like phenotypes with sensitivity to carbapenems and decreased piperacillin-tazobactam MICs, corroborating the data demonstrated for KPC-25 and KPC-40 (102, 116). Some mutants with deletions in the Ω-loop recovered susceptibility to all beta-lactam except ceftazidime and CAZavi thus becoming “hyper-specialized” on ceftazidime. The underlying mechanism is not yet known but could correspond to a trapping of ceftazidime.
FIG 6.
Antibiotic susceptibility of 16 antibiotics tested representing KPC-3, and the three different phenotypes resulting from mutations involved in ceftazidime-avibactam resistance illustrated with KPC-74, KPC-66 and KPC-29 expressed in E. coli TOP10. (AMX Amoxicillin, TIC Ticarcillin, FEP Cefepime, CEF Cefalotin, FOX Cefoxitin, CAZ Ceftazidime, AMC Co-amoxiclav, CTX Cefotaxime, PIP Piperacillin, ETP Ertapenem, ATM Aztreonam, PTZ Piperacillin-Tazobactam, FIX Cefixime, IMP imipenem, MERO Meropenem, and TEMO Temocillin).
Concerning the duplications in the 267–275 loop of KPC described in the literature (45, 82, 93, 98, 105–107, 117), although ertapenem and meropenem MICs decreased, they retained activity on imipenem revealing a versatile resistance of these mutants to carbapenems corresponding to a KPC-like phenotype (Fig. 6). The same observations of loss of hydrolytic activity have been highlighted, particularly for cephalosporins and aztreonam. Furthermore, the inhibition by tazobactam increases showing a greater decrease in IC50.
Mutants with deletions in loop 238–243 showed resistance to most penicillins and cephalosporins. They were highly inhibited by clavulanic acid and tazobactam, exhibiting an ESBL-like phenotype. All variants with a deletion within this loop lose their catalytic activities toward carbapenems (102).
MUTATION TRADE-OFF IN THE ENZYME STABILITY
From a thermodynamic point of view, folding and stability are closely related, and thermodynamic stability can be considered as the proportion of correctly folded proteins. In this sense, low stability corresponds to a significant proportion of misfolded proteins. Thus, a decrease in stability can be associated with a decrease in the number of functional proteins. Denatured proteins are controlled by degradation systems in bacteria, such as E. coli where it has been shown that the rate of degradation is higher for denatured proteins than for native proteins (91). Proteins of low stability therefore confer a reduced selective value; due to fewer functional proteins; significant energy costs and potentially other costs if the denatured proteins aggregate.
In class A beta-lactamases, substitutions close to the active site altering enzyme function are often associated with a cost in terms of protein stability. Mehta et al. show that simple substitutions near the active site; increasing the hydrolysis of ceftazidime; result in a loss of 2.6°C to 7°C (103). The V240G/H274Y mutant (KPC-8) has the greatest loss of stability among all the variants studied. Similarly, a significant decrease in stability was shown for variants with insertions, including duplications within the loop 267–275 far from the active site. Thus, increased hydrolysis of ceftazidime or CAZavi is associated with a cost in terms of stability for the protein.
KPC-2 and its variants are more stable than other Class A beta-lactamases. Indeed, the melting temperature of KPC-2 (66.5°C) is higher than that of TEM-1 (56.7°C) and CTX-M-14 (51°C) (78, 118). Despite the strong modifications caused by large insertions, stability remains important. This could explain the propensity of KPC to acquire these insertions, the ability to adapt to new substrates, including the CAZavi combination. The presence of a significant number of insertions/deletions in clinical variants of KPC is likely related to the high stability of the protein, making the structure permissive to these events.
Thus, the thermodynamic stability of the KPC enzyme compared to other class A beta-lactamases could explain their fascinating adaptability to the environment. In addition to their high tolerance to mutations, their evolutionary potential therefore remains unpredictable, and needs to be further evaluated.
CONCLUSION
The number of KPC variants resistant to CAZavi increased massively since its marketing, compromising the use of this antibiotic in the future. KPC shows significant adaptive potential with 126 clinical alleles deposited to date including 65 categorized as resistant to CAZavi. This adaptability is confirmed by numerous in vitro studies demonstrating this ability to adapt (47, 76, 87, 97).
Interestingly, this evolvability is associated with three mutation hot spots: Ω-loop, the loop 237–243, and the loop 266–275. The first two loops are associated with spectrum modification in all class A beta-lactamases, whereas the latter is specific to KPC. Both point mutations and indels were found in these loops and each structure exhibits a different mutational tolerance.
When KPC has a structural modification resulting in resistance to CAZavi, an enzymatic compromise emerges. Indeed, in the three loops described above, there is a decrease in carbapenemase activity with reductions in MIC for imipenem, meropenem and ertapenem. These changes are explained enzymologically by a reduction in kcat turnover and kcat/km catalytic efficiency.
KPC carbapenemases are remarkable for their significant proportion of insertions/deletions within in vivo isolates (41/65, 63%). As a comparison, only 4/145 (2.8%) of TEM variants harbor a deletion. The enzyme's ability to modify its structure and tolerate insertions/deletions reflects its plasticity and ability to adapt to new substrates. KPC has a very high melting temperature compared to TEM-1 or CTX-M (18, 103, 118) which remains high despite destabilization by substitutions, deletions or insertions. Thus, the propensity of KPC to acquire in vivo mutations conferring resistance to CAZavi, particularly via insertions and deletions, could therefore be related to its high natural stability (103).
Its high mutational tolerance and adaptability facing new substrates such as CAZavi, places beta-lactamase KPC as a real-speed evolutionary model.
The health care issue associated with this increased number of resistant mutants, motivated our research on the existing data in vivo, structural changes in the KPC enzyme, and their impact on resistance to CAZavi.
The evolutionary trade-off makes it difficult for beta-lactamase to resist both CAZavi and carbapenems revealing therapeutic options with, in particular, the new inhibitors meropenem-vaborbactam or imipenem-relebactam (17, 18, 20, 21, 23, 26, 90, 116). But here again, considering the adaptive potential of class A beta-lactamases, close monitoring of emerging resistance is necessary (11, 17, 116, 119).
ACKNOWLEDGMENTS
Claire Amaris Hobson is currently supported by funds from the ARC Foundation (Grant n°DOC20190509066) for a PhD, and the Q.E.M. team currently works with FRM fundings, EQU201903007848.
The authors have nothing to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Maltezou HC, Kontopidou F, Katerelos P, Daikos G, Roilides E, Theodoridou M. 2013. Infections caused by carbapenem-resistant Gram-negative pathogens in hospitalized children. Pediatr Infect Dis J 32:e151–e154. 10.1097/INF.0b013e3182804b49. [DOI] [PubMed] [Google Scholar]
- 2.Caselli D, Cesaro S, Fagioli F, Carraro F, Ziino O, Zanazzo G, Meazza C, Colombini A, Castagnola E, Infectious Diseases Study Group of the Italian Association of Pediatric Hematology and Oncology (AIEOP). 2016. Incidence of colonization and bloodstream infection with carbapenem-resistant Enterobacteriaceae in children receiving antineoplastic chemotherapy in Italy. Infect Dis 48:152–155. 10.3109/23744235.2015.1087647. [DOI] [PubMed] [Google Scholar]
- 3.Girmenia C, Rossolini GM, Piciocchi A, Bertaina A, Pisapia G, Pastore D, Sica S, Severino A, Cudillo L, Ciceri F, Scimè R, Lombardini L, Viscoli C, Rambaldi A, Gruppo Italiano Trapianto Midollo Osseo (GITMO). 2015. Infections by carbapenem-resistant Klebsiella pneumoniae in SCT recipients: a nationwide retrospective survey from Italy. Bone Marrow Transplant 50:282–288. 10.1038/bmt.2014.231. [DOI] [PubMed] [Google Scholar]
- 4.Freire MP, Pierrotti LC, Filho HHC, Ibrahim KY, Magri ASGK, Bonazzi PR, Hajar L, Diz MPE, Pereira J, Hoff PM, Abdala E. 2015. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae in cancer patients. Eur J Clin Microbiol Infect Dis 34:277–286. 10.1007/s10096-014-2233-5. [DOI] [PubMed] [Google Scholar]
- 5.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satlin MJ, Jenkins SG, Walsh TJ. 2014. The global challenge of carbapenem-resistant Enterobacteriaceae in transplant recipients and patients with hematologic malignancies. Clin Infect Dis 58:1274–1283. 10.1093/cid/ciu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase–producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 8.Martínez-Martínez L, González-López JJ. 2014. Carbapenemases in Enterobacteriaceae: types and molecular epidemiology. Enfermedades Infecc Microbiol Clínica 32:4–9. 10.1016/S0213-005X(14)70168-5. [DOI] [PubMed] [Google Scholar]
- 9.Palzkill T, Le QQ, Venkatachalam KV, LaRocco M, Ocera H. 1994. Evolution of antibiotic resistance: several different amino acid substitutions in an active site loop alter the substrate profile of beta-lactamase. Mol Microbiol 12:217–229. 10.1111/j.1365-2958.1994.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 10.Hansen GT. 2021. Continuous evolution: perspective on the epidemiology of carbapenemase resistance among Enterobacterales and other Gram-negative bacteria. Infect Dis Ther 10:75–92. 10.1007/s40121-020-00395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush K, Bradford PA. 2020. Epidemiology of β-lactamase-producing pathogens. Clin Microbiol Rev 33. 10.1128/CMR.00047-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798. 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naas T, Dortet L, Iorga BI. 2016. Structural and functional aspects of class A carbapenemases. Curr Drug Targets 17:1006–1028. 10.2174/1389450117666160310144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambler RP. 1980. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci 289:321–331. 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 15.Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. 2001. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151–1161. 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordmann P, Poirel L. 2019. Epidemiology and diagnostics of carbapenem resistance in Gram-negative bacteria. Clin Infect Dis 69:S521–S528. 10.1093/cid/ciz824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bush K, Bradford PA. 2019. Interplay between β-lactamases and new β-lactamase inhibitors. Nat Rev Microbiol 17:295–306. 10.1038/s41579-019-0159-8. [DOI] [PubMed] [Google Scholar]
- 18.Yahav D, Giske CG, Grāmatniece A, Abodakpi H, Tam VH, Leibovici L. 2020. New β-lactam-β-lactamase inhibitor combinations. Clin Microbiol Rev 34:e00115-20. 10.1128/CMR.00115-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagacé-Wiens PRS, Denisuik A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2013. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs 73:159–177. 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 20.Dhillon S. 2018. Meropenem/vaborbactam: a review in complicated urinary tract infections. Drugs 78:1259–1270. 10.1007/s40265-018-0966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Docquier J-D, Mangani S. 2018. An update on β-lactamase inhibitor discovery and development. Drug Resist Updat 36:13–29. 10.1016/j.drup.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez BA, Girotto JE, Nicolau DP. 2018. Ceftazidime/avibactam and ceftolozane/tazobactam: novel therapy for multidrug resistant gram negative infections in children. Curr Pediatr Rev 14:97–109. 10.2174/1573396314666180308150908. [DOI] [PubMed] [Google Scholar]
- 23.Tooke CL, Hinchliffe P, Bragginton EC, Colenso CK, Hirvonen VHA, Takebayashi Y, Spencer J. 2019. β-lactamases and β-lactamase inhibitors in the 21st century. J Mol Biol 431:3472–3500. 10.1016/j.jmb.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zasowski EJ, Rybak JM, Rybak MJ. 2015. The β-lactams strike back: ceftazidime-avibactam. Pharmacotherapy 35:755–770. 10.1002/phar.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini C, Menichetti F, Viscoli C, Campoli C, Venditti M, De Gasperi A, Mularoni A, Tascini C, Parruti G, Pallotto C, Sica S, Concia E, Cultrera R, De Pascale G, Capone A, Antinori S, Corcione S, Righi E, Losito AR, Digaetano M, Amadori F, Giacobbe DR, Ceccarelli G, Mazza E, Raffaelli F, Spanu T, Cauda R, Viale P. 2019. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis 68:355–364. 10.1093/cid/ciy492. [DOI] [PubMed] [Google Scholar]
- 26.Pogue JM, Bonomo RA, Kaye KS. 2019. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis 68:519–524. 10.1093/cid/ciy576. [DOI] [PubMed] [Google Scholar]
- 27.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aitken SL, Tarrand JJ, Deshpande LM, Tverdek FP, Jones AL, Shelburne SA, Prince RA, Bhatti MM, Rolston KVI, Jones RN, Castanheira M, Chemaly RF. 2016. High rates of nonsusceptibility to Ceftazidime-avibactam and identification of New Delhi metallo-β-lactamase production in Enterobacteriaceae bloodstream infections at a major cancer center. Clin Infect Dis 63:954–958. 10.1093/cid/ciw398. [DOI] [PubMed] [Google Scholar]
- 29.Endimiani A, Hujer AM, Perez F, Bethel CR, Hujer KM, Kroeger J, Oethinger M, Paterson DL, Adams MD, Jacobs MR, Diekema DJ, Hall GS, Jenkins SG, Rice LB, Tenover FC, Bonomo RA. 2009. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 63:427–437. 10.1093/jac/dkn547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodford N, Tierno PM, Young K, Tysall L, Palepou M-FI, Ward E, Painter RE, Suber DF, Shungu D, Silver LL, Inglima K, Kornblum J, Livermore DM. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class a β-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 48:4793–4799. 10.1128/AAC.48.12.4793-4799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castanheira M, Deshpande LM, Mendes RE, Canton R, Sader HS, Jones RN. 2019. Variations in the occurrence of resistance phenotypes and carbapenemase genes among Enterobacteriaceae isolates in 20 years of the SENTRY antimicrobial surveillance program. Open Forum Infect Dis 6:S23–S33. 10.1093/ofid/ofy347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee C-R, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. 2016. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol 7:895. 10.3389/fmicb.2016.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European Centre for Disease Prevention and Control. 2022. Antimicrobial resistance in the EU/EEA (EARS-net) - Annual Epidemiological Report 2020. Stockholm. ECDC. https://www.ecdc.europa.eu/sites/default/files/documents/ECDC-WHO-AMR-report.pdf. [Google Scholar]
- 34.Galani I, Karaiskos I, Karantani I, Papoutsaki V, Maraki S, Papaioannou V, Kazila P, Tsorlini H, Charalampaki N, Toutouza M, Vagiakou H, Pappas K, Kyratsa A, Kontopoulou K, Legga O, Petinaki E, Papadogeorgaki H, Chinou E, Souli M, Giamarellou H, On Behalf Of The Study Collaborators null. 2018. Epidemiology and resistance phenotypes of carbapenemase-producing Klebsiella pneumoniae in Greece, 2014 to 2016. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 23. 10.2807/1560-7917.ES.2018.23.30.1700775. [DOI] [PubMed] [Google Scholar]
- 35.Girmenia C, Serrao A, Canichella M. 2016. Epidemiology of carbapenem resistant Klebsiella pneumoniae infections in Mediterranean countries. Mediterr J Hematol Infect Dis 8:e2016032. 10.4084/MJHID.2016.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spyropoulou A, Papadimitriou-Olivgeris M, Bartzavali C, Vamvakopoulou S, Marangos M, Spiliopoulou I, Anastassiou ED, Christofidou M. 2016. A ten-year surveillance study of carbapenemase-producing Klebsiella pneumoniae in a tertiary care Greek university hospital: predominance of KPC- over VIM- or NDM-producing isolates. J Med Microbiol 65:240–246. 10.1099/jmm.0.000217. [DOI] [PubMed] [Google Scholar]
- 37.CNR Résistance aux antibiotiques. - Bilans d’activité. https://www.cnr-resistance-antibiotiques.fr/bilans-dactivite.html. Accessed 8 March 2022.
- 38.Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, Sahm DF, Bradford PA. 2016. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:1067–1078. 10.1128/AAC.02379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navon-Venezia S, Leavitt A, Schwaber MJ, Rasheed JK, Srinivasan A, Patel JB, Carmeli Y, Israeli KPC Kpn Study Group. 2009. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 53:818–820. 10.1128/AAC.00987-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazmierczak KM, Karlowsky JA, de Jonge BLM, Stone GG, Sahm DF. 2021. Epidemiology of carbapenem resistance determinants identified in meropenem-nonsusceptible Enterobacterales collected as part of a global surveillance program, 2012 to 2017. Antimicrob Agents Chemother 65:e0200020. 10.1128/AAC.02000-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adler A, Hussein O, Ben-David D, Masarwa S, Navon-Venezia S, Schwaber MJ, Carmeli Y, Setton E, Golan S, Brill S, Lipkin V, Frodin E, Mendelson G, Rave R, Yehuda N, Aizen I, Kaganski M, Gershkovich P, Sasson A, Yosef H, Stessman J, Zlatkin S, Or I, Lazary A, Weinberg I, Madjar J, Taichman S, Ben-Israel J, Vigder C, Bar’el C, Davidovitch Y, Charish L, on behalf of the Post-Acute-Care Hospital Carbapenem-Resistant Enterobacteriaceae Working Group. 2015. Persistence of Klebsiella pneumoniae ST258 as the predominant clone of carbapenemase-producing Enterobacteriaceae in post-acute-care hospitals in Israel, 2008–13. J Antimicrob Chemother 70:89–92. 10.1093/jac/dku333. [DOI] [PubMed] [Google Scholar]
- 43.Lam MMC, Wick RR, Watts SC, Cerdeira LT, Wyres KL, Holt KE. 2021. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat Commun 12:4188. 10.1038/s41467-021-24448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David S, Reuter S, Harris SR, Glasner C, Feltwell T, Argimon S, Abudahab K, Goater R, Giani T, Errico G, Aspbury M, Sjunnebo S, Feil EJ, Rossolini GM, Aanensen DM, Grundmann H, EuSCAPE Working Group, ESGEM Study Group. 2019. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol 4:1919–1929. 10.1038/s41564-019-0492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carattoli A, Arcari G, Bibbolino G, Sacco F, Tomolillo D, Di Lella FM, Trancassini M, Faino L, Venditti M, Antonelli G, Raponi G. 2021. Evolutionary trajectories toward ceftazidime-avibactam resistance in Klebsiella pneumoniae clinical isolates. Antimicrob Agents Chemother 65:e0057421. 10.1128/AAC.00574-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robledo IE, Aquino EE, Santé MI, Santana JL, Otero DM, León CF, Vázquez GJ. 2010. Detection of KPC in Acinetobacter spp. in Puerto Rico. Antimicrob Agents Chemother 54:1354–1357. 10.1128/AAC.00899-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nordmann P, Cuzon G, Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 9:228–236. 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 48.Köck R, Daniels-Haardt I, Becker K, Mellmann A, Friedrich AW, Mevius D, Schwarz S, Jurke A. 2018. Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: a systematic review. Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis 24:1241–1250. 10.1016/j.cmi.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Morakchi H, Loucif L, Gacemi-Kirane D, Rolain J-M. 2017. Molecular characterisation of carbapenemases in urban pigeon droppings in France and Algeria. J Glob Antimicrob Resist 9:103–110. 10.1016/j.jgar.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Vergara A, Pitart C, Montalvo T, Roca I, Sabaté S, Hurtado JC, Planell R, Marco F, Ramírez B, Peracho V, de Simón M, Vila J. 2017. Prevalence of extended-spectrum-β-lactamase- and/or carbapenemase-producing Escherichia coli isolated from yellow-legged gulls from Barcelona, Spain. Antimicrob Agents Chemother 61:e02071-16. 10.1128/AAC.02071-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiao J, Zhang Q, Alali WQ, Wang J, Meng L, Xiao Y, Yang H, Chen S, Cui S, Yang B. 2017. Characterization of extended-spectrum β-lactamases (ESBLs)-producing Salmonella in retail raw chicken carcasses. Int J Food Microbiol 248:72–81. 10.1016/j.ijfoodmicro.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 52.Ahlstrom CA, Ramey AM, Woksepp H, Bonnedahl J. 2019. Repeated detection of carbapenemase-producing Escherichia coli in gulls inhabiting Alaska. Antimicrob Agents Chemother 63:e00758-19. 10.1128/AAC.00758-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mani Y, Mansour W, Mammeri H, Denamur E, Saras E, Boujâafar N, Bouallègue O, Madec J-Y, Haenni M. 2017. KPC-3-producing ST167 Escherichia coli from mussels bought at a retail market in Tunisia. J Antimicrob Chemother 72:2403–2404. 10.1093/jac/dkx124. [DOI] [PubMed] [Google Scholar]
- 54.de Araujo CFM, Silva DM, Carneiro MT, Ribeiro S, Fontana-Maurell M, Alvarez P, Asensi MD, Zahner V, Carvalho-Assef APD. 2016. Detection of carbapenemase genes in aquatic environments in Rio de Janeiro, Brazil. Antimicrob Agents Chemother 60:4380–4383. 10.1128/AAC.02753-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. 2000. The protein data bank. Nucleic Acids Res 28:235–242. 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ke W, Bethel CR, Thomson JM, Bonomo RA, van den Akker F. 2007. Crystal structure of KPC-2: insights into carbapenemase activity in class A beta-lactamases. Biochemistry 46:5732–5740. 10.1021/bi700300u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galdadas I, Lovera S, Pérez-Hernández G, Barnes MD, Healy J, Afsharikho H, Woodford N, Bonomo RA, Gervasio FL, Haider S. 2018. Defining the architecture of KPC-2 carbapenemase: identifying allosteric networks to fight antibiotics resistance. Sci Rep 8. 10.1038/s41598-018-31176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galdadas I, Qu S, Oliveira ASF, Olehnovics E, Mack AR, Mojica MF, Agarwal PK, Tooke CL, Gervasio FL, Spencer J, Bonomo RA, Mulholland AJ, Haider S. 2021. Allosteric communication in class A β-lactamases occurs via cooperative coupling of loop dynamics. Elife 10:e66567. 10.7554/eLife.66567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Philippon A, Slama P, Dény P, Labia R. 2016. A structure-based classification of class A β-lactamases, a broadly diverse family of enzymes. Clin Microbiol Rev 29:29–57. 10.1128/CMR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oueslati S, Tlili L, Exilie C, Bernabeu S, Iorga B, Bonnin RA, Dortet L, Naas T. 2020. Different phenotypic expression of KPC β-lactamase variants and challenges in their detection. J Antimicrob Chemother 75:769–771. 10.1093/jac/dkz508. [DOI] [PubMed] [Google Scholar]
- 61.Papp-Wallace KM, Taracila M, Wallace CJ, Hujer KM, Bethel CR, Hornick JM, Bonomo RA. 2010. Elucidating the role of Trp105 in the KPC-2 β-lactamase. Protein Sci 19:1714–1727. 10.1002/pro.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soeung V, Lu S, Hu L, Judge A, Sankaran B, Prasad BVV, Palzkill T. 2020. A drug-resistant β-lactamase variant changes the conformation of its active-site proton shuttle to alter substrate specificity and inhibitor potency. J Biol Chem 295:18239–18255. 10.1074/jbc.RA120.016103. [DOI] [PubMed] [Google Scholar]
- 63.Tooke CL, Hinchliffe P, Bonomo RA, Schofield CJ, Mulholland AJ, Spencer J. 2021. Natural variants modify Klebsiella pneumoniae carbapenemase (KPC) acyl–enzyme conformational dynamics to extend antibiotic resistance. J Biol Chem 296:100126. 10.1074/jbc.RA120.016461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Humphries RM, Yang S, Hemarajata P, Ward KW, Hindler JA, Miller SA, Gregson A. 2015. First report of ceftazidime-avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae iolate. Antimicrob Agents Chemother 59:6605–6607. 10.1128/AAC.01165-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Wang J, Wang R, Cai Y. 2020. Resistance to ceftazidime-avibactam and underlying mechanisms. J Glob Antimicrob Resist 22:18–27. 10.1016/j.jgar.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 66.Di Bella S, Giacobbe DR, Maraolo AE, Viaggi V, Luzzati R, Bassetti M, Luzzaro F, Principe L. 2021. Resistance to ceftazidime/avibactam in infections and colonisations by KPC-producing Enterobacterales: a systematic review of observational clinical studies. J Glob Antimicrob Resist 25:268–281. 10.1016/j.jgar.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Venditti C, Butera O, Meledandri M, Balice MP, Cocciolillo GC, Fontana C, D'Arezzo S, De Giuli C, Antonini M, Capone A, Messina F, Nisii C, Di Caro A. 2021. Molecular analysis of clinical isolates of ceftazidime-avibactam-resistant Klebsiella pneumoniae. Clin Microbiol Infect 27:1040.e1–1040.e6. 10.1016/j.cmi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Palzkill T. 2018. Structural and mechanistic basis for extended-spectrum drug-resistance mutations in altering the specificity of TEM, CTX-M, and KPC β-lactamases. Front Mol Biosci 5:16. 10.3389/fmolb.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petrosino JF, Palzkill T. 1996. Systematic mutagenesis of the active site omega loop of TEM-1 beta-lactamase. J Bacteriol 178:1821–1828. 10.1128/jb.178.7.1821-1828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Furey IM, Mehta SC, Sankaran B, Hu L, Prasad BVV, Palzkill T. 2021. Local interactions with the Glu166 base and the conformation of an active site loop play key roles in carbapenem hydrolysis by the KPC-2 β-lactamase. J Biol Chem 296:100799. 10.1016/j.jbc.2021.100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barnes MD, Winkler ML, Taracila MA, Page MG, Desarbre E, Kreiswirth BN, Shields RK, Nguyen M-H, Clancy C, Spellberg B, Papp-Wallace KM, Bonomo RA. 2017. Klebsiella pneumoniae carbapenemase-2 (KPC-2), substitutions at ambler position Asp179, and resistance to ceftazidime-avibactam: unique antibiotic-resistant phenotypes emerge from β-lactamase protein engineering. mBio 8. 10.1128/mBio.00528-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salverda MLM, De Visser JAGM, Barlow M. 2010. Natural evolution of TEM-1 β-lactamase: experimental reconstruction and clinical relevance. FEMS Microbiol Rev 34:1015–1036. 10.1111/j.1574-6976.2010.00222.x. [DOI] [PubMed] [Google Scholar]
- 73.Levitt PS, Papp-Wallace KM, Taracila MA, Hujer AM, Winkler ML, Smith KM, Xu Y, Harris ME, Bonomo RA. 2012. Exploring the role of a conserved class A residue in the Ω-Loop of KPC-2 β-lactamase: a mechanism for ceftazidime hydrolysis. J Biol Chem 287:31783–31793. 10.1074/jbc.M112.348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winkler ML, Papp-Wallace KM, Bonomo RA. 2015. Activity of ceftazidime/avibactam against isogenic strains of Escherichia coli containing KPC and SHV β-lactamases with single amino acid substitutions in the Ω-loop. J Antimicrob Chemother 70:2279–2286. 10.1093/jac/dkv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sampson JM, Ke W, Bethel CR, Pagadala SRR, Nottingham MD, Bonomo RA, Buynak JD, van den Akker F. 2011. Ligand-dependent disorder of the Omega loop observed in extended-spectrum SHV-type beta-lactamase. Antimicrob Agents Chemother 55:2303–2309. 10.1128/AAC.01360-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haidar G, Clancy CJ, Shields RK, Hao B, Cheng S, Nguyen MH. 2017. Mutations in blaKPC-3 that confer ceftazidime-Aaibactam resistance encode novel KPC-3 variants that function as extended-spectrum β-lactamases. Antimicrob Agents Chemother 61:e02534-16. 10.1128/AAC.02534-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zawadzke LE, Chen CC, Banerjee S, Li Z, Wäsch S, Kapadia G, Moult J, Herzberg O. 1996. Elimination of the hydrolytic water molecule in a class A beta-lactamase mutant: crystal structure and kinetics. Biochemistry 35:16475–16482. 10.1021/bi962242a. [DOI] [PubMed] [Google Scholar]
- 78.Brown NG, Shanker S, Prasad BVV, Palzkill T. 2009. Structural and biochemical evidence that a TEM-1 beta-lactamase N170G active site mutant acts via substrate-assisted catalysis. J Biol Chem 284:33703–33712. 10.1074/jbc.M109.053819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hemarajata P, Humphries RM. 2019. Ceftazidime/avibactam resistance associated with L169P mutation in the omega loop of KPC-2. J Antimicrob Chemother 74:1241–1243. 10.1093/jac/dkz026. [DOI] [PubMed] [Google Scholar]
- 80.Venditti C, Nisii C, Ballardini M, Meledandri M, Di Caro A. 2019. Identification of L169P mutation in the omega loop of KPC-3 after a short course of ceftazidime/avibactam. J Antimicrob Chemother 74:2466–2467. 10.1093/jac/dkz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cano Á, Guzmán-Puche J, García-Gutiérrez M, Castón JJ, Gracia-Ahufinger I, Pérez-Nadales E, Recio M, Natera AM, Marfil-Pérez E, Martínez-Martínez L, Torre-Cisneros J. 2020. Use of carbapenems in the combined treatment of emerging ceftazidime/avibactam-resistant and carbapenem-susceptible KPC-producing Klebsiella pneumoniae infections: report of a case and review of the literature. J Glob Antimicrob Resist 22:9–12. 10.1016/j.jgar.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 82.Göttig S, Frank D, Mungo E, Nolte A, Hogardt M, Besier S, Wichelhaus TA. 2019. Emergence of ceftazidime/avibactam resistance in KPC-3-producing Klebsiella pneumoniae in vivo. J Antimicrob Chemother 74:3211–3216. 10.1093/jac/dkz330. [DOI] [PubMed] [Google Scholar]
- 83.Guzmán-Puche J, Pérez-Nadales E, Pérez-Vázquez M, Causse M, Gracia-Ahufinger I, Mendez-Natera A, Allalou-Ruiz Y, Elías C, Oteo-Iglesias J, Torre-Cisneros J, Martínez-Martínez L. 2022. In vivo selection of KPC-94 and KPC-95 in Klebsiella pneumoniae isolates from patients treated with ceftazidime/avibactam. Int J Antimicrob Agents 59:106524. 10.1016/j.ijantimicag.2022.106524. [DOI] [PubMed] [Google Scholar]
- 84.Krishnan NP, Nguyen NQ, Papp-Wallace KM, Bonomo RA, van den Akker F. 2015. Inhibition of Klebsiella β-lactamases (SHV-1 and KPC-2) by avibactam: a structural study. PLoS One 10:e0136813. 10.1371/journal.pone.0136813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alsenani TA, Viviani SL, Kumar V, Taracila MA, Bethel CR, Barnes MD, Papp-Wallace KM, Shields RK, Nguyen MH, Clancy CJ, Bonomo RA, van den Akker F. 2022. Structural characterization of the D179N and D179Y variants of KPC-2 β-lactamase: Ω-loop destabilization as a mechanism of resistance to ceftazidime-avibactam. Antimicrob Agents Chemother 66:e0241421. 10.1128/aac.02414-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jousset AB, Oueslati S, Emeraud C, Bonnin RA, Dortet L, Iorga BI, Naas T. 2021. KPC-39-mediated resistance to ceftazidime-avibactam in a Klebsiella pneumoniae ST307 clinical isolate. Antimicrob Agents Chemother 65:e0116021. 10.1128/AAC.01160-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: a case report and review of literature. Open Forum Infect Dis 4. 10.1093/ofid/ofx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giddins MJ, Macesic N, Annavajhala MK, Stump S, Khan S, McConville TH, Mehta M, Gomez-Simmonds A, Uhlemann A-C. 2018. Successive emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in blaKPC-2-harboring Klebsiella pneumoniae sequence type 307 isolates. Antimicrob Agents Chemother 62:e02101-17. 10.1128/AAC.02101-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. 2018. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother 62:e02497-17. 10.1128/AAC.02497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsivkovski R, Lomovskaya O. 2020. Potency of vaborbactam is less affected than that of avibactam in strains producing KPC-2 mutations that confer resistance to ceftazidime-avibactam. Antimicrob Agents Chemother 64:e01936-19. 10.1128/AAC.01936-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Compain F, Arthur M. 2017. Impaired inhibition by avibactam and resistance to the ceftazidime-avibactam combination due to the D179Y substitution in the KPC-2 β-lactamase. Antimicrob Agents Chemother 61:e00451-17. 10.1128/AAC.00451-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Antonelli A, Giani T, Di Pilato V, Riccobono E, Perriello G, Mencacci A, Rossolini G. 2019. KPC-31 expressed in a ceftazidime/avibactam-resistant Klebsiella pneumoniae is associated with relevant detection issues. J Antimicrob Chemother 74:2464–2466. 10.1093/jac/dkz156. [DOI] [PubMed] [Google Scholar]
- 93.Sun L, Chen W, Li H, Li L, Zou X, Zhao J, Lu B, Li B, Wang C, Li H, Liu Y, Cao B. 2020. Phenotypic and genotypic analysis of KPC-51 and KPC-52, two novel KPC-2 variants conferring resistance to ceftazidime/avibactam in the KPC-producing Klebsiella pneumoniae ST11 clone background. J Antimicrob Chemother 75:3072–3074. 10.1093/jac/dkaa241. [DOI] [PubMed] [Google Scholar]
- 94.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. In vitro selection of meropenem resistance among ceftazidime-avibactam-resistant, meropenem-susceptible Klebsiella pneumoniae isolates with variant KPC-3 carbapenemases. Antimicrob Agents Chemother 61:e00079-17. 10.1128/AAC.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gaibani P, Campoli C, Lewis RE, Volpe SL, Scaltriti E, Giannella M, Pongolini S, Berlingeri A, Cristini F, Bartoletti M, Tedeschi S, Ambretti S. 2018. In vivo evolution of resistant subpopulations of KPC-producing Klebsiella pneumoniae during ceftazidime/avibactam treatment. J Antimicrob Chemother 73:1525–1529. 10.1093/jac/dky082. [DOI] [PubMed] [Google Scholar]
- 96.Taracila MA, Bethel CR, Hujer AM, Papp-Wallace KM, Barnes MD, Rutter JD, VanPelt J, Shurina BA, van den Akker F, Clancy CJ, Nguyen MH, Cheng S, Shields RK, Page RC, Bonomo RA. 2022. Different conformations revealed by NMR underlie resistance to ceftazidime/avibactam and susceptibility to meropenem and imipenem among D179Y variants of KPC β-lactamase. Antimicrob Agents Chemother 66:e0212421. 10.1128/aac.02124-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Livermore DM, Warner M, Jamrozy D, Mushtaq S, Nichols WW, Mustafa N, Woodford N. 2015. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother 59:5324–5330. 10.1128/AAC.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang L, Shen W, Zhang R, Cai J. 2022. Identification of a novel ceftazidime-avibactam-resistant KPC-2 variant, KPC-123, in Citrobacter koseri following ceftazidime-avibactam treatment. Front Microbiol 13:930777. 10.3389/fmicb.2022.930777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petrella S, Ziental-Gelus N, Mayer C, Renard M, Jarlier V, Sougakoff W. 2008. Genetic and structural insights into the dissemination potential of the extremely broad-spectrum class A beta-lactamase KPC-2 identified in an Escherichia coli strain and an Enterobacter cloacae strain isolated from the same patient in France. Antimicrob Agents Chemother 52:3725–3736. 10.1128/AAC.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hidalgo-Grass C, Warburg G, Temper V, Benenson S, Moses AE, Block C, Strahilevitz J. 2012. KPC-9, a novel carbapenemase from clinical specimens in Israel. Antimicrob Agents Chemother 56:6057–6059. 10.1128/AAC.01156-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Papp-Wallace KM, Taracila M, Hornick JM, Hujer AM, Hujer KM, Distler AM, Endimiani A, Bonomo RA. 2010. Substrate selectivity and a novel role in inhibitor discrimination by residue 237 in the KPC-2 beta-lactamase. Antimicrob Agents Chemother 54:2867–2877. 10.1128/AAC.00197-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hobson CA, Bonacorsi S, Jacquier H, Choudhury A, Magnan M, Cointe A, Bercot B, Tenaillon O, Birgy A. 2020. KPC beta-lactamases are permissive to insertions and deletions conferring substrate spectrum modifications and resistance to ceftazidime-avibactam. Antimicrob Agents Chemother 64:e01175-20. 10.1128/AAC.01175-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mehta SC, Rice K, Palzkill T. 2015. Natural variants of the KPC-2 carbapenemase have evolved increased catalytic efficiency for ceftazidime hydrolysis at the cost of enzyme stability. PLoS Pathog 11:e1004949. 10.1371/journal.ppat.1004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oueslati S, Iorga B, Tlili L, Exilie C, Zavala A, Dortet L, Jousset A, Bernabeu S, Bonnin R, Naas T. 2019. Unravelling ceftazidime/avibactam resistance of KPC-28, a KPC-2 variant lacking carbapenemase activity. J Antimicrob Chemother 74:2239–2246. 10.1093/jac/dkz209. [DOI] [PubMed] [Google Scholar]
- 105.Räisänen K, Koivula I, Ilmavirta H, Puranen S, Kallonen T, Lyytikäinen O, Jalava J. 2019. Emergence of ceftazidime-avibactam-resistant Klebsiella pneumoniae during treatment, Finland, December 2018. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 24. 10.2807/1560-7917.ES.2019.24.19.1900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mueller L, Masseron A, Prod’Hom G, Galperine T, Greub G, Poirel L, Nordmann P. 2019. Phenotypic, biochemical and genetic analysis of KPC-41, a KPC-3 variant conferring resistance to ceftazidime-avibactam and exhibiting reduced carbapenemase activity. Antimicrob Agents Chemother 63:e01111-19. 10.1128/AAC.01111-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Poirel L, Vuillemin X, Juhas M, Masseron A, Bechtel-Grosch U, Tiziani S, Mancini S, Nordmann P. 2020. KPC-50 confers resistance to ceftazidime-avibactam associated with reduced carbapenemase activity. Antimicrob Agents Chemother 64:e00321-20. 10.1128/AAC.00321-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wolter DJ, Kurpiel PM, Woodford N, Palepou M-FI, Goering RV, Hanson ND. 2009. Phenotypic and enzymatic comparative analysis of the novel KPC variant KPC-5 and its evolutionary variants, KPC-2 and KPC-4. Antimicrob Agents Chemother 53:557–562. 10.1128/AAC.00734-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bunzel HA, Anderson JLR, Mulholland AJ. 2021. Designing better enzymes: insights from directed evolution. Curr Opin Struct Biol 67:212–218. 10.1016/j.sbi.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 111.Banerjee S, Pieper U, Kapadia G, Pannell LK, Herzberg O. 1998. Role of the omega-loop in the activity, substrate specificity, and structure of class A beta-lactamase. Biochemistry 37:3286–3296. 10.1021/bi972127f. [DOI] [PubMed] [Google Scholar]
- 112.Liao Q, Kulkarni Y, Sengupta U, Petrović D, Mulholland AJ, van der Kamp MW, Strodel B, Kamerlin SCL. 2018. Loop motion in triosephosphate isomerase is not a simple open and shut case. J Am Chem Soc 140:15889–15903. 10.1021/jacs.8b09378. [DOI] [PubMed] [Google Scholar]
- 113.Wang X, Minasov G, Shoichet BK. 2002. The structural bases of antibiotic resistance in the clinically derived mutant beta-lactamases TEM-30, TEM-32, and TEM-34. J Biol Chem 277:32149–32156. 10.1074/jbc.M204212200. [DOI] [PubMed] [Google Scholar]
- 114.Munoz-Price LS, Reeme AE, Buchan BW, Mettus RT, Mustapha MM, Van Tyne D, Shields RK, Doi Y. 2019. Patient-to-patient transmission of Klebsiella pneumoniae carbapenemase variants with reduced ceftazidime-avibactam susceptibility. Antimicrob Agents Chemother 63:e00955-19. 10.1128/AAC.00955-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y, Delmas J, Sirot J, Shoichet B, Bonnet R. 2005. Atomic resolution structures of CTX-M beta-lactamases: extended spectrum activities from increased mobility and decreased stability. J Mol Biol 348:349–362. 10.1016/j.jmb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 116.Hobson CA, Cointe A, Jacquier H, Choudhury A, Magnan M, Courroux C, Tenaillon O, Bonacorsi S, Birgy A. 2021. Cross-resistance to cefiderocol and ceftazidime-avibactam in KPC β-lactamase mutants and the inoculum effect. Clin Microbiol Infect 27:1172.e7–1172.e10. 10.1016/j.cmi.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 117.Lebreton F, Corey BW, McElheny CL, Iovleva A, Preston L, Margulieux KR, Cybulski RJ, Mc Gann P, Doi Y, Bennett JW. 2021. Characterization of KPC-82, a KPC-2 variant conferring resistance to ceftazidime-avibactam in a carbapenem non-susceptible, clinical isolate of Citrobacter koseri. Antimicrob Agents Chemother 65:e00150-21. 10.1128/AAC.00150-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brown NG, Pennington JM, Huang W, Ayvaz T, Palzkill T. 2010. Multiple global suppressors of protein stability defects facilitate the evolution of extended-spectrum TEM β-lactamases. J Mol Biol 404:832–846. 10.1016/j.jmb.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bianco G, Boattini M, Comini S, Iannaccone M, Bondi A, Cavallo R, Costa C. 2022. In vitro activity of cefiderocol against ceftazidime-avibactam susceptible and resistant KPC-producing Enterobacterales: cross-resistance and synergistic effects. Eur J Clin Microbiol Infect Dis 41:63–70. 10.1007/s10096-021-04341-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S3. Download aac.00447-22-s0001.pdf, PDF file, 0.3 MB (344KB, pdf)