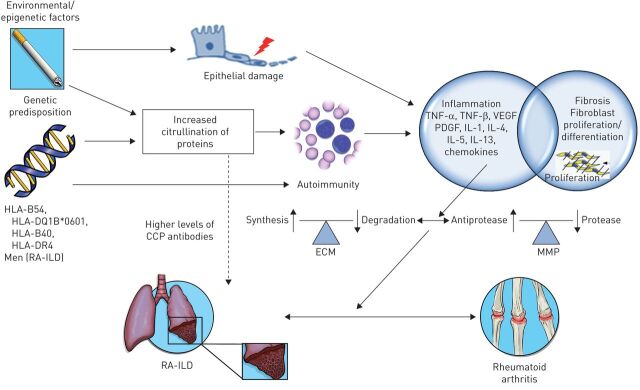
FIGURE 1.
Schematic illustration of the concepts in the pathogenesis of rheumatoid arthritis associated-interstitial lung disease (RA-ILD). While the pathogenesis of RA-ILD is not fully understood, genetic predisposition and epigenetic factors are thought to play a role. Some patients with rheumatoid arthritis may have a genetic predisposition to ILD. Human leukocyte antigen (HLA)-B54, HLA-DQ1B*0601, HLA-B40, HLA-DR4 and the site encoding α-1 protease inhibitor have been associated with increased risk of ILD in rheumatoid arthritis, while some shared epitopes (HLA-DRB1) have been associated with the development of rheumatoid arthritis but decreased rates of RA-ILD. Male sex and older age are additional risk factors for RA-ILD. Both genetic factors and smoking are associated with increased citrullination of proteins in the lung, which allows for exposure of new epitopes and an autoimmune response. Higher levels of anti-cyclic citrullinated peptide (CCP) antibodies have been found in patients with RA-ILD compared to patients with rheumatoid arthritis only, but the role of such antibodies in the pathogenesis of RA-ILD is not clear. Smoking is also a risk factor for rheumatoid arthritis alone and results in repeated injuries to the alveolar epithelium and alterations in the cytokine milieu. As in other ILDs, the inflammatory response activates cytokines, chemokines and growth factors, such as tumor necrosis factor (TNF), vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF) and interleukins (IL). These contribute to a differentiation and proliferation of fibroblasts, increased synthesis and deposition of extracellular matrix (ECM) and increased activity of matrix metalloproteinases (MMP) resulting in ILD. Fibroblasts in the synovial lining play a similar role in the pathogenesis of joint manifestations of rheumatoid arthritis. (Figure prepared with assistance from Sean Mclaughlin; Seattle, WA, USA).