Abstract
Background:
Palbociclib is a selective inhibitor of CDK4/6 approved in metastatic breast cancer as well as evidence of activity in malignancies with CDK4-amplified. Extensive preclinical evidence has demonstrated synergy of CDK4/6 inhibitors with platinum chemotherapy suggesting a potential role for clinical synthetic lethality. Given the sensitivity to platinum therapy as well as the landscape of genomic alterations, concurrent treatment with platinum chemotherapy and palbociclib is of significant interest as a novel treatment approach.
Patients and Methods:
Patients with unresectable, recurrent, or metastatic head and neck cancer (R/M HNC) were enrolled. Eligible patients were required to have no previous treatment with cytotoxic chemotherapy in the recurrent/metastatic setting. This was a multicenter phase II trial in which patients were administered carboplatin in addition to concurrent palbociclib. The primary endpoint of this trial was 12-week disease control rate (DCR).
Results:
Twenty-one patients were enrolled and 18 were evaluable for response. Grade 3/4 treatment related toxicities were seen in 79% of patients of which the most common were related to myelosuppression. 12-week DCR was 33% (5 patients with stable disease, 1 with a partial response). Median progression free survival was 2.9 months (range: 1.2-13.3) and overall survival was 4.6 months (range: 1.4-14.8).
Conclusion:
The combination of carboplatin and palbociclib is associated with significant treatment related toxicity and insufficient anti-tumor activity.
Keywords: head and neck neoplasm, palbociclib, CDK4/6, carboplatin, metastatic head and neck cancer, HNSCC
Introduction
Unresectable recurrent or metastatic head and neck cancer (R/M HNC) has a dismal survival and development of targeted therapeutics has been met with little success. Platinum compounds have been the backbone of therapy with a response rate (RR) of 10-30% to single agent regimens[1,2]. Until recently, first line chemotherapy consisted of the EXTREME regimen which has a demonstrated RR of 36% and OS of 10.1 months[3]. Given the limited performance status at diagnosis and underlying co-morbidities of many patients with head and neck cancers, some are not candidates for this triplet regimen, thus new therapeutic strategies are needed. Targeted therapies offer many potential benefits in the treatment of other cancers. However, unlike non-small cell lung adenocarcinoma or melanoma, characterization of the genomic landscape of head and neck squamous cell carcinoma has failed to elucidate a driver mutation[4].
Now with a greater understanding of aberrant cellular signaling and function in carcinogenesis, there has been a shift in modern chemotherapeutics towards targeted therapies specific to the molecular drivers of each distinct malignancy. Commonly altered genes include cell cycle regulatory genes CDKN2A and CCND1 (Cyclin D1)[5]. These cell cycle regulatory genes encode a complex group of proteins including cyclin dependent kinases and cyclin D which regulate progression through the cell cycle. Sustained CDK4/6 activation is believed to be present in the majority of tumors as a mechanism to continually allow transition from G1 to S phase[6]. In R/M HNSCC, alterations in CDKN2A or CCND1 are two genomic events which lead to loss of checkpoint control. In patients with R/M HNC CDKN2A alterations include homozygous deletions in 30%, additional gene mutations in 10-20%, and epigenetic alterations leading to inactivation in up to 80%. CCND1 has been noted to be amplified as part of the 11q13 amplicon in about 20% of cases[4,5,7,8]. Between the two, genomic alterations in either CDKN2A and CCND1 have been identified in between 60-94% of R/M HNC making these potential targets of interest[4,5,9].
Palbociclib is a reversible, oral, highly selective inhibitor of CDK4 and CDK6 (CDK 4/6) approved in hormone receptor positive metastatic breast cancer[10,11]. Recognizing the potential as a targeted therapy in other cancers with CDK abnormalities, palbociclib has been evaluated in advanced CDK4-amplified liposarcoma and resulted in an improvement in progression free survival[12]. This study suggests activity in diverse malignancies with cyclin-dependent kinase dysregulation.
Palbociclib has been evaluated in human papilloma virus negative (HPV-) R/M HNC in combination with cetuximab and found to result in tumor reduction in 39% of patients[13,14]. Preclinical studies evaluating CDK 4/6 inhibitors have suggested synergy with chemotherapy in several cancer types. These combination effects were demonstrated to be due to chemotherapy induced tumor S-Phase recruitment/synchronization resulting in a greater degree of cell cycle arrest and apoptosis[15]. A preclinical study evaluating ribociclib and cisplatin in ovarian cancer models demonstrated significant synergy with concurrent administration with reduction in stem-like cells in both Rb mutant and wild type cell lines[16]. Preclinical work in head and neck squamous cell lines has similarly demonstrated suggested synergistic potential of palbociclib and platinum therapy.
We conducted a multi-institution phase II trial to investigate the clinical activity of combining carboplatin and palbociclib in patients with R/M HNC. Our hypothesis was that combination treatment with carboplatin plus palbociclib (CarPal) would have significant synergy in eliciting an antitumor response and hence achieve an improved 12-week disease control rate (DCR) compared to single agent chemotherapy.
Methods
Patient eligibility
This was a phase 2 multicenter open label trial approved by the Institutional Review Board (IRBMED) of the University of Michigan Rogel Cancer Center (NCT03194373). All patients provided written informed consent. Patients ≥ 18 years old with histologically documented progressive squamous cell head and neck cancer non amenable to curative treatment were eligible. All patients had the presence of measurable disease by CT scan, an ECOG performance status of 0-2, and a life expectancy of ≥12 weeks. Patients had to have adequate hematopoietic, hepatic, and renal function defined as: absolute neutrophil count ≥ 1.5x109 cell/ml, platelets ≥75,000 cells/mm3, hemoglobin ≥ 9.0 g/dL, concentrations of total serum bilirubin within 1.5x the upper limit of normal (ULN), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) within 2.5x institutional upper limits of normal unless there were liver metastases in which case AST and ALT within 5.0 x ULN, serum creatinine clearance ≥ 30 ml/min). Women of childbearing potential must have had a negative serum or urine pregnancy test within 3 days prior to treatment.
Eligible patients were required to have no previous treatment with cytotoxic chemotherapy in the recurrent/metastatic setting however, previous treatment with non-cytotoxic agents and/or concurrent chemoradiation in the recurrent/metastatic setting was permitted. Patients were excluded if they had gastrointestinal abnormalities causing impaired absorption precluding administration of medications by mouth or feeding tube. Additional exclusionary criteria included evidence of untreated or progressive brain metastases, an uncontrolled medical disorder or active infection which would impair their ability to receive study treatment, or history of severe allergic reaction to either cisplatin or carboplatin.
Treatment plan
Enrolled patients underwent a complete history and physical examination, baseline laboratory studies (CBC with differential, comprehensive metabolic profile), and radiographic staging studies (CT Neck/Chest and others as clinically warranted) prior to treatment initiation. All screening assessments were completed within 28 days prior to the start of treatment. A pretreatment biopsy of the metastatic tumor was required if clinically safe and feasible for correlative studies analyses.
Carboplatin was given on Day 1 with a starting dose of AUC=5 and palbociclib was administered 125 mg daily days 1-14. Cycle length was 21 days. Premedications, antiemetics, and G-CSF were administered at the discretion of the treating physician. Therapy was continued for a total of six 21 day cycles of treatment or until unacceptable toxicity/disease progression occurred. If an ongoing response was noted after six cycles, patients were transitioned to single agent palbociclib. Maintenance palbociclib was dosed at 125 mg daily, days 1-21 of a 28-day cycle, and continued until disease progression or intolerance.
Evaluation of response
Imaging studies for evaluation of response of target radiologic lesions were performed after two cycles from treatment initiation and continued at 6 week intervals. If a patient proceeded to maintenance therapy, imaging was obtained every 9 weeks from cycle 7, day 1. Target lesions were followed on each imaging study and analyzed primarily by following the sum of the largest diameter of all target lesions. Radiologic response was assessed according to RECIST v1.1.
Statistical considerations
Treatment-related adverse events were graded according to the Common Terminology for Adverse Events version 4.0 (CTCAE v4). Treatment response was evaluated by Response Evaluation Criteria in Solid Tumors (RECIST version 1.0). Patients were considered evaluable for response if they underwent response evaluation imaging after 2 cycles of receiving both carboplatin and palbociclib. Best overall response was defined as the best response achieved from treatment initiation to progression or death. Disease control rate (DCR) included complete response (CR), partial response (PR) or stable disease (SD). Disease control rate at 12 weeks was presented with 95% confidence intervals using an exact binomial distribution for calculation of the standard error. Overall survival was the defined as the time from study enrollment to death from any cause. Data were censored at the last follow-up for patients who were alive at the time of analysis. Progression-free survival was defined as the time from study enrollment until disease progression or death. Median survival times were computed using the Kaplan-Meier method with standard error computed using Greenwood’s formula. All analyses were done using SAS 9.4 software.
Targeted Exome Sequencing
From the clinical cohort, 9 patients had sufficient tumor biopsy material for targeted next generation sequencing. Thus, DNA was isolated from FFPE blocks using the Qiagen AllPrep kit as previously described[17] and advanced for NGS if it met our previously defined quality standards defined by Qubit and Bioanalyzer analysis[18,19]. Thus, DNA that met our quality control standards was used to prepare sequencing libraries with the Rubicon Thruplex kit according to the manufacturer’s instructions. We performed a custom capture using a bait set manufactured by Nextera consisting of high density probes for 224 genes, using the Nextera Rapid Capture Custom Enrichment Kit (Catalogue #: FC-140-1008). The genes targeted in this panel are listed in Supplemental Table 1. We then barcoded and pooled the custom capture libraries for sequencing to an average of >100x depth on an Illumina HiSEQ4000 with paired end 150nt sequencing.
Targeted Exome Variant calling
Prior to analysis, the quality of the sequencing reads was defined using FastQC v.0.11.5. Trim galore v0.4.5 was then used to trim reads containing sequencing adapters as previously described by our team[20,21]. Using BWA v0.7.15, the processed reads were then aligned to the hg19 reference genome. We next used PicardTools v2.4.1 to sort, deduplicate and index the mapped reads and base quality score recalibration was performed using GATK v3.6 to generate clean aligned reads for variant calling. For each tumor-normal pair in the set, we used Samtools v1.9 to create pileup files for analysis. Then, using the somatic mode of the variant caller Varscan v2.4.1, we called variants from these mpileup files and annotated them using Goldex Helix Varseq v2.1.0. This was followed by filtering the variants in the introns and intergenic regions. We used a minimum threshold of 5 reads supporting the alternate allele in the tumor samples were considered as potential positive high confidence calls, as previously described[22].
Copy Number Analysis
Copy number estimation calls were also performed using the pre-processed tumor-normal BAM files with the Aberration Detection in Tumor Exome (ADTEx) v.2.0 algorithm as described[23]. This software package assigns five copy number states from 0 to 4 based on its estimated copy number. State 0 represents a homozygous deletion, state 1 corresponds to a heterozygous deletion, normal copy number is denoted by state 2, while states 3 and 4 correspond to a copy gain and amplification, respectively. Heatmaps for the copy number data were made using ComplexHeatmap v2.0.0.
Variant Effect Scoring
We used CRAVAT 5.2.4 (Cancer-Related Analysis of Variants Toolkit) for variant impact scoring. This web-based tool supports several analysis programs for the interpretation of genomic variants. One of the programs provided by the toolkit, VEST v4.0, was used to assign pathogenicity scores to the variants and CHASM v3.1 was used for somatic missense variant scoring.
Results
Patient characteristics
Twenty-one patients were enrolled, of which 18 were evaluable for response. All 19 enrolled patients who received at least one cycle of carboplatin and palbociclib were included for toxicity analysis. The baseline characteristics of all enrolled patients are summarized in Table 1. The median age was 66.5 years (range: 40-74). The most common primary site of disease was oropharyngeal cancer (6 patients, 33%) and most patients were ECOG performance status 1 (56%). p16 status, a surrogate for human papilloma virus (HPV) infection, was available for 12 patients of which the majority were p16 negative (8 patients, 44%). Nine patients had previously received platinum agents as part of the management of their locally advanced head and neck cancer. Seven patients (39%) had their last dose of platinum chemotherapy at least 180 days prior to study treatment, whereas and two (11%) had platinum chemotherapy in the last 180 days. Two patients (11%) had been treated with PD-1 inhibitors for R/M disease prior to enrollment.
Table 1: Patient Demographics and Clinical Characteristics.
This table displays the demographics of the patients included in analysis for efficacy
| Age | n | 18 |
| Mean | 62.7 years | |
| Median (range) | 66.5 (40-74) | |
|
| ||
| Gender, n (%) | Male | 16 |
| Female | 2 | |
|
| ||
| ECOG Performance Status, n(%) | 0 (Fully functional) | 8 |
| 1 (Minor Impairment) | 10 | |
|
| ||
| Disease Primary Site | Oral Cavity | 5 |
| Oropharynx | 6 | |
| Larynx | 4 | |
| Nasopharynx | 2 | |
| Other | 1 | |
|
| ||
| Disease State | Locally advanced/recurrent | 2 |
| Metastatic | 16 | |
|
| ||
| T Stage | T1 | 1 |
| T2 | 3 | |
| T3 | 2 | |
| T4 | 7 | |
| Tx | 5 | |
|
| ||
| N Stage | N0 | 5 |
| N1 | 3 | |
| N2 | 6 | |
| Nx | 4 | |
|
| ||
| M Stage | M0 | 2 |
| M1 | 16 | |
|
| ||
| p16 | Positive | 4 |
| Negative | 8 | |
| not done | 6 | |
|
| ||
| Previous Exposure to Immunotherapy | 2 | |
|
| ||
| Previous Exposure to Platinum | 9 | |
| Platinum exposure ≤180 days | 2 | |
| Platinum exposure >180 days | 7 | |
Toxicity
The mean duration of therapy was 4.8 cycles (range: 2-17). Combinatorial therapy with carboplatin and palbociclib was encountered by significant toxicity. Grade 3 or greater toxicities were seen in 79% of all patients with the most common being myelosuppression (Table 2). 2 patients discontinued therapy due to toxicity and 8 patients (44%) required dose modifications most commonly for hematologic toxicities (Table 3). Severe hematologic toxicities included neutropenia (10 patients, 56%), anemia (7 patients, 39%), thrombocytopenia (3 patients, 17%), and febrile neutropenia in one patient. Other than hematologic toxicity, severe toxicities seen in greater than 10% of the population included fatigue (5 patients, 28%) and nausea (2 patients, 11%).
Table 2: Treatment Related Toxicities.
This table lists the toxicities observed in patients treated with carboplatin and palbociclib for HNSCC
| Toxicity | Grade 1 or 2 | Grade 3 or 4 | Total |
|---|---|---|---|
| Neutrophil count decreased | 2 (11%) | 10 (56%) | 12 (67%) |
| Anemia | 5 (28%) | 7 (39%) | 12 (67%) |
| White blood cell decreased | 3 (17%) | 7 (39%) | 10 (56%) |
| Fatigue | 7 (39%) | 5 (28%) | 12 (67%) |
| Platelet count decreased | 4 (22%) | 3 (17%) | 7 (39%) |
| Nausea | 6 (33%) | 2 (11%) | 8 (44%) |
| Vomiting | 2 (11%) | 1 (5%) | 3 (16%) |
| Weight loss | 4 (22%) | 0 | 4 (22%) |
| Febrile neutropenia | 0 | 1 (5%) | 1 (5%) |
Table 3: Treatment Tolerability.
This table lists the characteristics associated with tolerability of carboplatin and palbociclib including duration of therapy and reason for discontinuation.
| n | 18 | |
|
| ||
| Duration of Treatment | ||
| # Cycles completed | Mean | 4.8 |
| Median (range) | 4 (2-17) | |
| Carboplatin Dose Modifications, n | ||
| 0 | 9 | |
| 1 | 8 | |
| 2+ | 1 | |
|
| ||
| Reason for treatment discontinuation, n | ||
| Adverse Event | 1 | |
| Disease Progression | 15 | |
| Patient Discretion | 1 | |
| Other | 1 | |
Efficacy
Two patients stopped treatment prior to response evaluation. One patient was not started on therapy after enrollment due to abrupt decline in performance status, another received carboplatin but never received palbociclib. A third patient was removed from study prior to imaging per physician discretion. A 12-week disease control rate of 33% (95% CI: 13-59) was observed of which 5 patients (28%) had stable disease and 1 patient (6%) had a partial response (Table 4). Two patients were noted to have stable disease at 6 weeks however died before the 12-week response assessment. Degree of tumor response is depicted in the waterfall plot in Figure 1. The median progression free survival (PFS) was 2.9 months (range: 1.2-13.3) with a 6-month progression free survival (PFS) of 22% (95% CI: 7-43). One exceptional responder was noted who had stable disease for 17 cycles (Figure 2). The median overall survival was 4.6 months (range: 1.4-14.8) (Figure 3).
Table 4: Treatment Efficacy.
This table describes the treatment efficacy and patient outcomes
| n=18 | |
|---|---|
| Median PFS, days (range) | 89 (36-406) |
| 2.9 months | |
|
| |
| 6 month PFS, % (95% CI)1 | 22% (7%,43%) |
|
| |
| Median OS, days (range2) | 142 (43-453) |
| 4.6 months | |
|
| |
| Best Overall Response Evaluation | |
| Progressive Disease (PD), n (%) | 10 |
| Stable Disease (SD), n (%) | 7 |
| Partial Response (PR), n (%) | 1 |
|
| |
| 6 Week PFS, % | 44% |
|
| |
| 12 Week Disease Control Rate (SD+PR+CR), % (95% CI) | 33% (13%,59%) |
6 month PFS and 95% confidence interval estimated using life-table method.
Denotes that the highest observation was censored at maximum
Fig. 1.
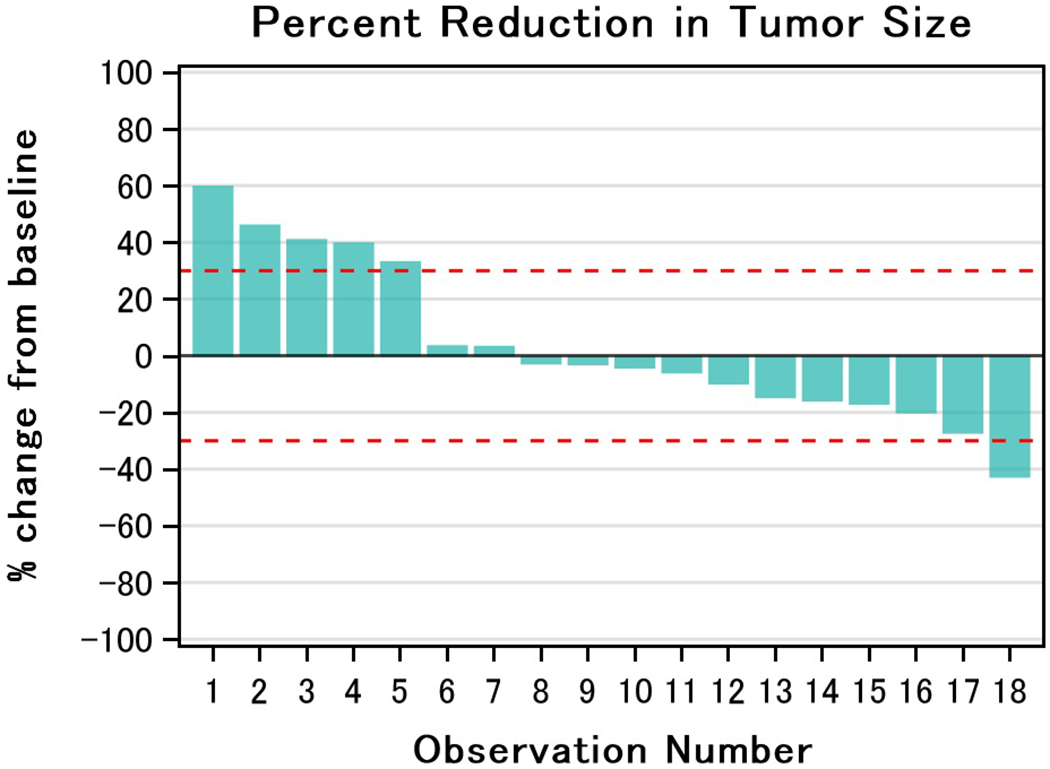
Degree of Tumor Response. This waterfall plot demonstrates the maximal degree of response to treatment amongst evaluable patients
Fig. 2.
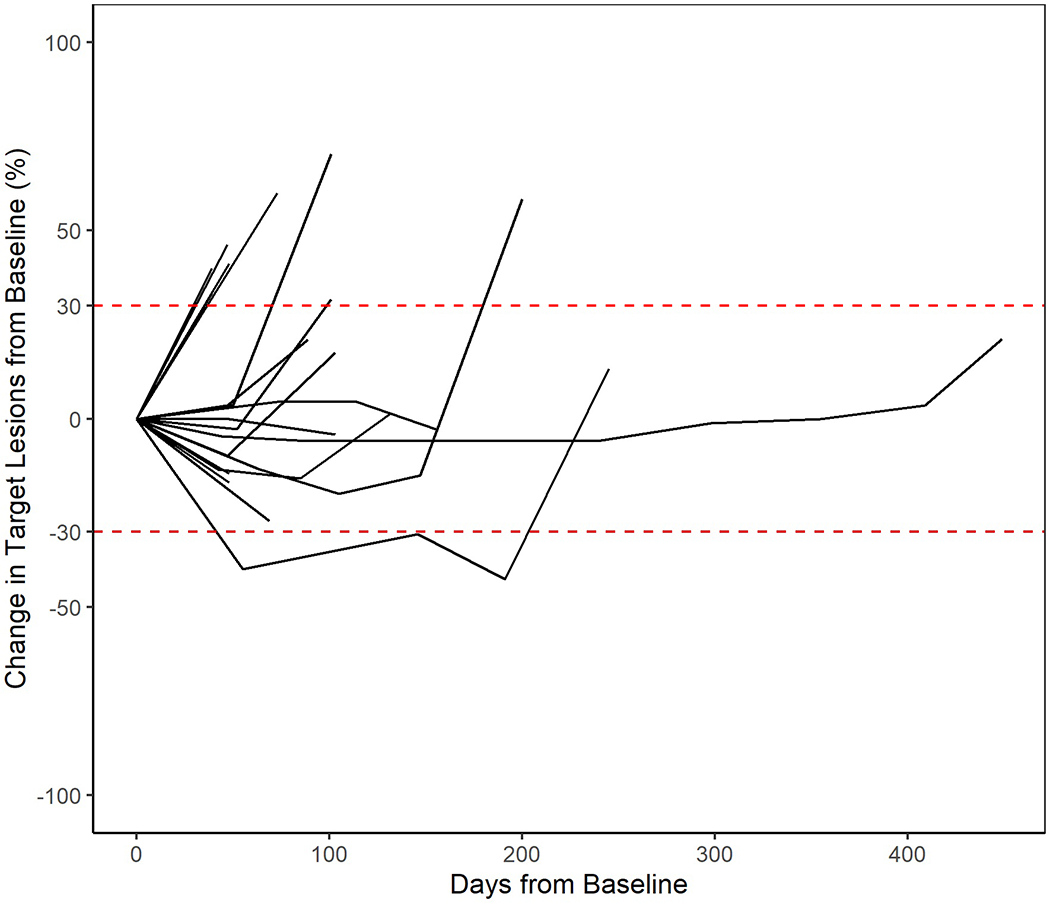
Duration of Tumor Response. This figure the degree of radiographic response over time in patients treated with carboplatin and palbociclib.
Fig. 3.
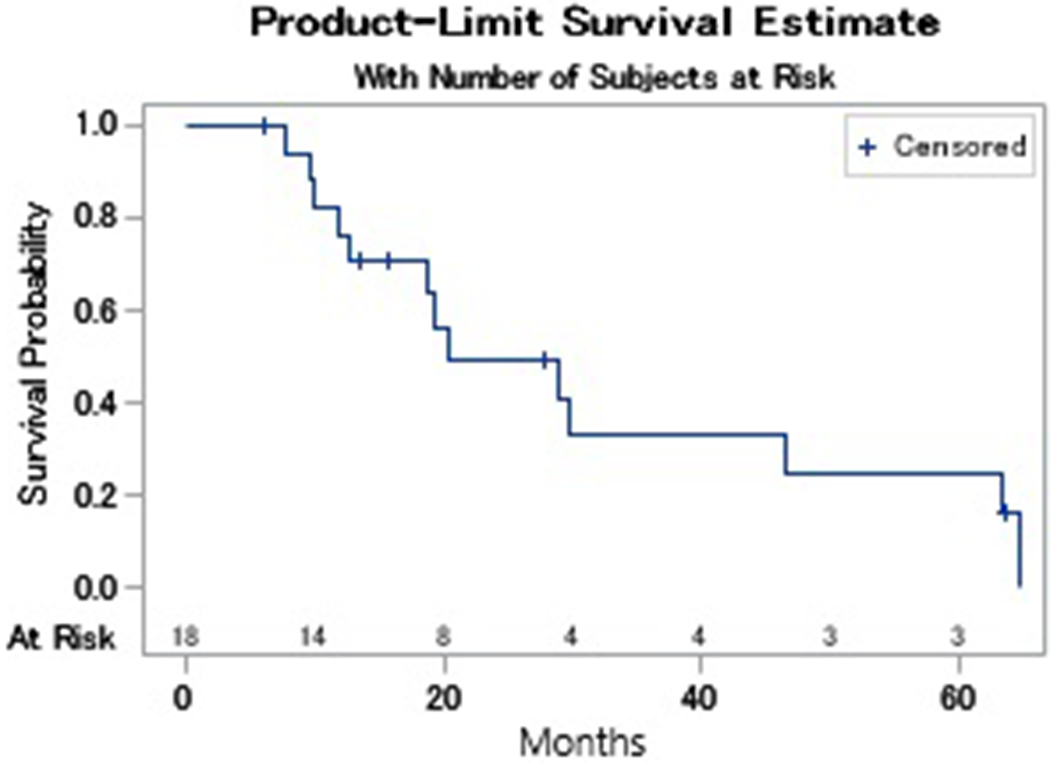
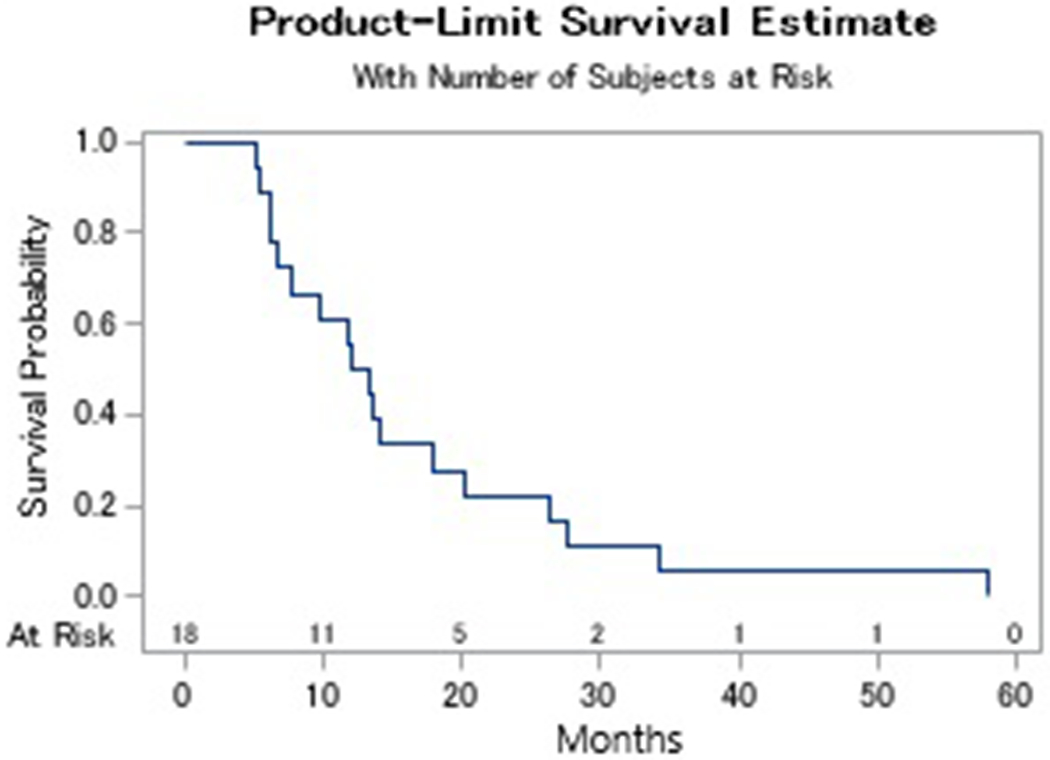
Kaplan Meier Survival Analysis. This figure illustrates the overall survival (Fig 3a) and progression free survival (Fig 3b) amongst patients treated with carboplatin and palbociclib
Correlative studies
To define genomic changes to commonly mutated HNSCC genes, we sequenced targeted capture based NGS libraries from 9 of the tumors in this cohort to an average of >100X depth (Supplemental Table 2). This approach discovered copy number changes to CDKN2A, CDKN2B and RB1 in several of the analyzed tumors, but no mutations in these genes (Figure 4). Specific molecular alterations from each tumor along with their corresponding functional annotation scores are listed in Supplemental Figure 1. We also observed that 4/6 (66%) of p16 negative tumors harbored TP53 mutations consistent with expected TP53 mutation rates. Surprisingly, we also discovered that 2/9 (22%) tumors harbored BRAF alterations, including a E501K mutation, potentially suggesting that these two tumors did not respond to the therapy because they were dependent on alternative drivers. Pathogenecity scores were calculated for each of the somatic mutations, which supported a predicted oncogenic role of BRAF (Supplemental Table 3).
Fig. 4:
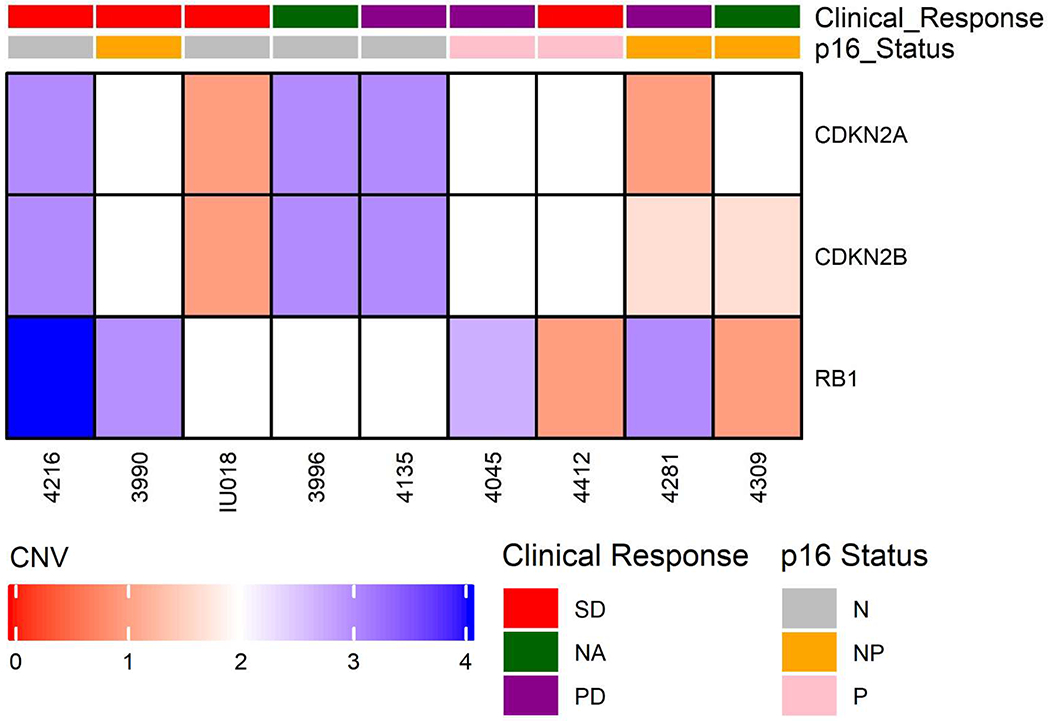
Genomic Alterations and Clinical Characteristics. This focused heat map characterizes clinical response to therapy, clinical characteristics, and selected results of targeted sequencing. Abbreviations: Clinical Response: SD- Stable disease, PD- progressive disease, NA- Not evaluable for response. p16 status: N- Negative, P- Positive, NP- Not performed.
Discussion
This multicenter phase II trial is the first to evaluate the clinical activity of combining a cytotoxic chemotherapy and CDK 4/6 inhibitor in R/M HNC and, to the best of our knowledge, the first to report in any malignancy. This study demonstrates that despite preclinical evidence, the combination of carboplatin and palbociclib is not an active regimen in R/M HNC and is associated with significant toxicities.
The genomic landscape has been well characterized in head and neck cancer. Although there is a lack of driver mutations, frequent alterations of cell cycle regulatory genes and the availability of CDK 4/6 inhibitors have made these of particular interest for targeted therapy. Furthermore, combination with cytotoxic chemotherapy has the potential to target the cancer stem-like cell population, potentiate the impact of platinum, and have activity in Rb mutant and wild type populations[16]. The prospective role for synergy using palbociclib in R/M HNC has been highlighted with recent publications demonstrating evidence of activity when combined with cetuximab[13,14].
In our study, responses were seen in both platinum and cetuximab refractory populations regardless of HPV status. This has further supported the hope that incorporation of targeted therapy with first line chemotherapy may result in tumor control. Despite this supportive evidence, we failed to demonstrate treatment outcomes that were equal to or greater than historical controls. There are numerous possible reasons may account for these poor responses to CarPal. However clinically, patients were primarily HPV negative (at least 8 patients, up to 14). Previous studies have shown less than 10% of non-oropharyngeal squamous cell carcinomas are HPV positive[24,25], hence we postulate the majority of the patients not tested for HPV were negative. HPV negative disease has repeatedly been associated with decreased response rates to therapy regardless of class or line of therapy[26–28].
Interestingly, in our limited correlative analysis no association was noted between response to therapy and either HPV status or somatic alterations (including CDKN2A, CDKN2B and RB1). Based on its mechanism, palbociclib would be thought to be of no benefit in patients with HPV related cancers as they would be expected to have intact Rb but in fact the exceptional responder who remained on treatment for 17 cycles had HPV related oropharyngeal disease with just this molecular phenotype. Although our conclusions are limited given a small sample size and lack of a control arm, previous data supports the activity of palbociclib in HPV related cancers[13] as well in Rb deficient tumors[16].
Biologically, it could be postulated that CDK 4/6 inhibition could have synchronized cells in a G1 arrest leading to G0 dormancy. In G0 dormancy, concurrent cytotoxic chemotherapy would be of little utility given the lack of cellular growth or replication. However, studies evaluating dosing strategies have found concurrent inhibition of CDK 4/6 and platinum potentiates cytotoxicity[15,16,29]. Given the lack of samples after treatment initiation, it is not possible to assess the mechanism of palbociclib in this study. Emerging evidence suggests that CDK 4/6 inhibitors have activity beyond the G1/S checkpoint including induction of dephosphorylation of ATM and CHK1 as well as modulating the immune microenvironment[30]. As such, there are rationale for future trials evaluating the combination of a CDK 4/6 and checkpoint inhibitor.
Toxicity is of significant concern in our patient population, especially given the somewhat limited pretreatment performance status in some, primarily due to pre-existing co-morbidities. Grade 3 or 4 toxicities are common occurrences with conventional therapies seen in 76% of patients treated with cytotoxic doublets and 82% of patients treated with the combination of platinum, fluorouracil, and cetuximab[3]. With targeted therapy, the goal is hopefully to lower rates toxicities compared to traditional cytotoxic regimens. This is of particular interest for those not candidates for conventional triple agent therapy. This study demonstrated marked rates of toxicities on par with multi-drug cytotoxic regimens. Furthermore, patients discontinued therapy due to toxicities and significant issues with nausea, vomiting, and weight loss. As treatment is palliative in intent, any toxicity which diminishes quality of life must be considered. Since the initiation of this study, single agent pembrolizumab has been approved in this patient population in patients with PD-L1 CPS ≥ 1%[31]. Pembrolizumab now offers the promise of not only improved survival but also decreased toxicities compared to standard of care therapies.
In conclusion, concurrent treatment with palbociclib and carboplatin has insufficient antitumor activity in R/M HNC. The lack of efficacy may be due to the underlying patient/tumor characteristics; however, the induction of chemotherapy resistant G0 dormancy cannot be ruled out. Furthermore, significant treatment related toxicities were seen, limiting patient tolerance and impairing quality of life. Synergy may be seen between palbociclib and other classes of agents, possibly immunotherapy. Additional studies are needed as best how to potentially incorporate palbociclib into the treatment of patients with locally advanced, recurrent SCCHN.
Supplementary Material
Supplemental Table 1: Targeted Gene Panel
This table lists genes included with high density coverage in our head and neck targeted panel.
Supplemental Table 2: Targeted Sequencing of Patient Cohort
This oncoplot illustrates the most commonly altered genes in our cohort.
Supplemental Table 3: Genomic Variants and Pathogenicity Scores
This table lists the genomic variants and calculated pathogenicity scores.
Supplemental Fig. 1: Tumor Molecular Alterations and Functional Annotation Scores. This heat map characterizes clinical response to therapy, clinical characteristics, and full results of targeted sequencing. Abbreviations: Clinical Response: SD- Stable disease, PD- progressive disease, NA- Not evaluable for response. p16 status: N- Negative, P- Positive, NP- Not performed.
Funding
Pfizer Inc provided an independent research grant for this trial. A.B. and J.C.B. received partial support from ACS grant: 132034-RSG-18-062-01-TBG.
Footnotes
Conflict of Interest
All authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
CRediT Classification:
Conceptualization: Paul Swiecicki, Chad Brenner, Francis Worden
Data curation: Apurva Bhangale, Emily Bellile, Paul Swiecicki
Formal analysis: Apurva Bhangale, Chad Brenner, Emily Bellile, Paul Swiecicki
Methodology: Chad Brenner, Emily Bellile, Paul Swiecicki
Resources: Chad Brenner, Paul Swiecicki, Francis Worden, Greg Durm
Writing - original draft: Paul Swiecicki, Emily Bellile, Apurva Bhangale, Chad Brenner
Writing - review and editing: Paul Swiecicki, Emily Bellile, Chad Brenner, Francis Worden, Greg Durm
References
- 1.Jacobs C, Lyman G, Velez-Garcia E, Sridhar KS, Knight W, Hochster H, Goodnough LT, Mortimer JE, Einhorn LH, Schacter L, et al. : A phase iii randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. Journal of clinical oncology : official journal of the American Society of Clinical Oncology (1992) 10(2):257–263. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Metch B, Schuller DE, Ensley JF, Hutchins LF, Triozzi P, Kish JA, McClure S, VonFeldt E, Williamson SK, et al. : Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: A southwest oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology (1992) 10(8):1245–1251. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F et al. : Platinum-based chemotherapy plus cetuximab in head and neck cancer. The New England journal of medicine (2008) 359(11):1116–1127. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas N: Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature (2015) 517(7536):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riaz N, Morris LG, Lee W, Chan TA: Unraveling the molecular genetics of head and neck cancer through genome-wide approaches. Genes & diseases (2014) 1(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi YJ, Anders L: Signaling through cyclin d-dependent kinases. Oncogene (2014) 33(15):1890–1903. [DOI] [PubMed] [Google Scholar]
- 7.van der Riet P, Nawroz H, Hruban RH, Corio R, Tokino K, Koch W, Sidransky D: Frequent loss of chromosome 9p21-22 early in head and neck cancer progression. Cancer research (1994) 54(5):1156–1158. [PubMed] [Google Scholar]
- 8.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y et al. : The cbio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer discovery (2012) 2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, Zhao M, Ortega Alves MV, Chang K, Drummond J, Cortez E et al. : Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer discovery (2013) 3(7):770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, Toogood PL: Specific inhibition of cyclin-dependent kinase 4/6 by pd 0332991 and associated antitumor activity in human tumor xenografts. Molecular cancer therapeutics (2004) 3(11):1427–1438. [PubMed] [Google Scholar]
- 11.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y et al. : The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, her2-negative, advanced breast cancer (paloma-1/trio-18): A randomised phase 2 study. The Lancet Oncology (2015) 16(1):25–35. [DOI] [PubMed] [Google Scholar]
- 12.Dickson MA, Tap WD, Keohan ML, D’Angelo SP, Gounder MM, Antonescu CR, Landa J, Qin LX, Rathbone DD, Condy MM, Ustoyev Y et al. : Phase ii trial of the cdk4 inhibitor pd0332991 in patients with advanced cdk4-amplified well-differentiated or dedifferentiated liposarcoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology (2013) 31(16):2024–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michel L, Ley J, Wildes TM, Schaffer A, Robinson A, Chun SE, Lee W, Lewis J Jr., Trinkaus K, Adkins D: Phase i trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Oral oncology (2016) 58(41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adkins D, Ley J, Neupane P, Worden F, Sacco AG, Palka K, Grilley-Olson JE, Maggiore R, Salama NN, Trinkaus K, Van Tine BA et al. : Palbociclib and cetuximab in platinum-resistant and in cetuximab-resistant human papillomavirus-unrelated head and neck cancer: A multicentre, multigroup, phase 2 trial. The Lancet Oncology (2019) 20(9):1295–1305. [DOI] [PubMed] [Google Scholar]
- 15.Pishvaian MJYS, El Zouhairi M, et al. : Abstract 5047: Synergistic anti-cancer activity of the cdk4/6 inhibitor pd-0332991 in combination with 5-fluorouracil-based chemotherapy in human colon cancer cells. Cancer research (2010) 70(8 Suppl):Abstract nr 5047. [Google Scholar]
- 16.Iyengar M, O’Hayer P, Cole A, Sebastian T, Yang K, Coffman L, Buckanovich RJ: Cdk4/6 inhibition as maintenance and combination therapy for high grade serous ovarian cancer. Oncotarget (2018) 9(21):15658–15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birkeland AC, Foltin SK, Michmerhuizen NL, Hoesli RC, Rosko AJ, Byrd S, Yanik M, Nor JE, Bradford CR, Prince ME, Carey TE et al. : Correlation of crtc1/3-maml2 fusion status, grade and survival in mucoepidermoid carcinoma. Oral oncology (2017) 68(5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birkeland AC, Yanik M, Tillman BN, Scott MV, Foltin SK, Mann JE, Michmerhuizen NL, Ludwig ML, Sandelski MM, Komarck CM, Carey TE et al. : Identification of targetable erbb2 aberrations in head and neck squamous cell carcinoma. JAMA otolaryngology-- head & neck surgery (2016) 142(6):559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tillman BN, Yanik M, Birkeland AC, Liu CJ, Hovelson DH, Cani AK, Palanisamy N, Carskadon S, Carey TE, Bradford CR, Tomlins SA et al. : Fibroblast growth factor family aberrations as a putative driver of head and neck squamous cell carcinoma in an epidemiologically low-risk patient as defined by targeted sequencing. Head & neck (2016) 38 Suppl 1(E1646–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann JE, Kulkarni A, Birkeland AC, Kafelghazal J, Eisenberg J, Jewell BM, Ludwig ML, Spector ME, Jiang H, Carey TE, Brenner JC: The molecular landscape of the university of michigan laryngeal squamous cell carcinoma cell line panel. Head & neck (2019) 41(9):3114–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwig ML, Kulkarni A, Birkeland AC, Michmerhuizen NL, Foltin SK, Mann JE, Hoesli RC, Devenport SN, Jewell BM, Shuman AG, Spector ME et al. : The genomic landscape of um-scc oral cavity squamous cell carcinoma cell lines. Oral oncology (2018) 87(144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birkeland AC, Burgin SJ, Yanik M, Scott MV, Bradford CR, McHugh JB, McLean SA, Sullivan SE, Nor JE, McKean EL, Brenner JC: Pathogenetic analysis of sinonasal teratocarcinosarcomas reveal actionable beta-catenin overexpression and a beta-catenin mutation. Journal of neurological surgery Part B, Skull base (2017) 78(4):346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith J, Kulkarni A, Birkeland AC, McHugh JB, Brenner JC: Whole-exome sequencing of sinonasal small cell carcinoma arising within a papillary schneiderian carcinoma in situ. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery (2018) 159(5):859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung CH, Zhang Q, Kong CS, Harris J, Fertig EJ, Harari PM, Wang D, Redmond KP, Shenouda G, Trotti A, Raben D et al. : P16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology (2014) 32(35):3930–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zafereo ME, Xu L, Dahlstrom KR, Viamonte CA, El-Naggar AK, Wei Q, Li G, Sturgis EM: Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral oncology (2016) 56(47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H et al. : Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine (2010) 363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenthal DI, Harari PM, Giralt J, Bell D, Raben D, Liu J, Schulten J, Ang KK, Bonner JA: Association of human papillomavirus and p16 status with outcomes in the imcl-9815 phase iii registration trial for patients with locoregionally advanced oropharyngeal squamous cell carcinoma of the head and neck treated with radiotherapy with or without cetuximab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology (2016) 34(12):1300–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F et al. : Nivolumab for recurrent squamous-cell carcinoma of the head and neck. The New England journal of medicine (2016) 375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luke JJ, D’Adamo DR, Dickson MA, Keohan ML, Carvajal RD, Maki RG, de Stanchina E, Musi E, Singer S, Schwartz GK: The cyclin-dependent kinase inhibitor flavopiridol potentiates doxorubicin efficacy in advanced sarcomas: Preclinical investigations and results of a phase i dose-escalation clinical trial. Clinical cancer research : an official journal of the American Association for Cancer Research (2012) 18(9):2638–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, Ivanova E et al. : Cdk4/6 inhibition augments antitumor immunity by enhancing t-cell activation. Cancer discovery (2018) 8(2):216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr., Psyrri A, Baste N, Neupane P, Bratland A, Fuereder T et al. : Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (keynote-048): A randomised, open-label, phase 3 study. Lancet (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Targeted Gene Panel
This table lists genes included with high density coverage in our head and neck targeted panel.
Supplemental Table 2: Targeted Sequencing of Patient Cohort
This oncoplot illustrates the most commonly altered genes in our cohort.
Supplemental Table 3: Genomic Variants and Pathogenicity Scores
This table lists the genomic variants and calculated pathogenicity scores.
Supplemental Fig. 1: Tumor Molecular Alterations and Functional Annotation Scores. This heat map characterizes clinical response to therapy, clinical characteristics, and full results of targeted sequencing. Abbreviations: Clinical Response: SD- Stable disease, PD- progressive disease, NA- Not evaluable for response. p16 status: N- Negative, P- Positive, NP- Not performed.


