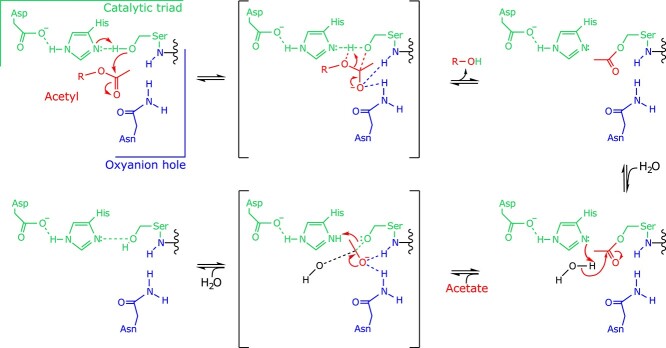
Fig. 2.
The general SGNH hydrolase mechanism of action. The nucleophilic attack of the ester carbonyl (red) is accomplished by the action of a Ser-His-Asp triad (green), and the resulting oxyanion intermediate species is stabilized by a positively charged pocket often referred to as the oxyanion hole (blue), coupled with the release of the alcohol coproduct. A covalent acetyl-enzyme intermediate is formed during the catalytic cycle, which is then attacked by a water molecule to generate the acetate coproduct and free enzyme.