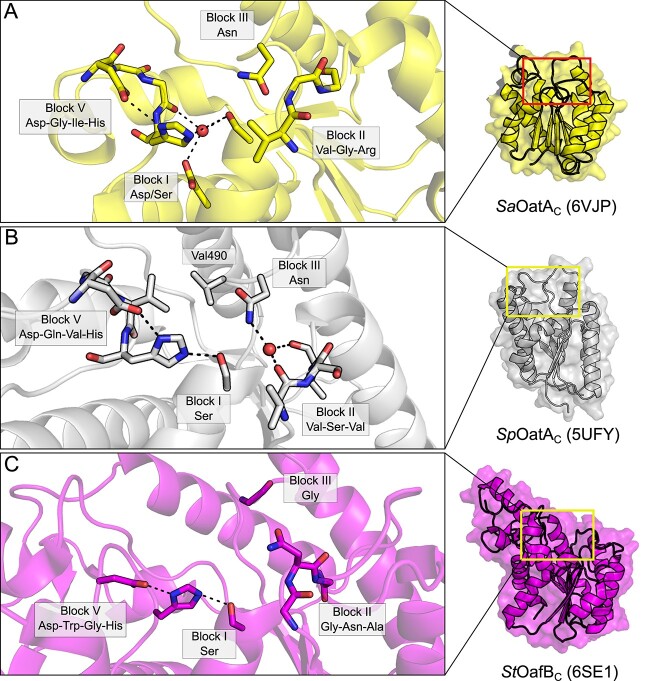
Fig. 8.
The SGNH-AT3 family. (A) The active site of the S. aureus OatA C-terminal domain (SaOatAC; 6VJP; yellow) also contains a highly ordered water molecule but coordinated instead by a Block I Asp/Ser pair and a carbonyl group from the Block V loop, which limits solvent access to the active site pocket and promotes transferase activity. (B) The active site of the S. pneumoniae OatA C-terminal domain (SpOatAC; 5UFY; grey) promotes transferase activity through a highly ordered water molecule at the active site; however, this is achieved through coordination by an inverted Block II loop unique to Streptococci, along with the conserved Block III Asn. Additionally, two Val residues presented by the Block III and V loops form a hydrophobic wall that further limits solvent access to this pocket. (C) The Salmonella ser. Paratyphi O-antigen acetyltransferase OafB (StOafBC; 6SE1; magenta) possesses an additional helical element and a structured linker domain, which constrain the solvent-accessible surface area of the domain and conferring specificity for acceptor co-substrate(s).