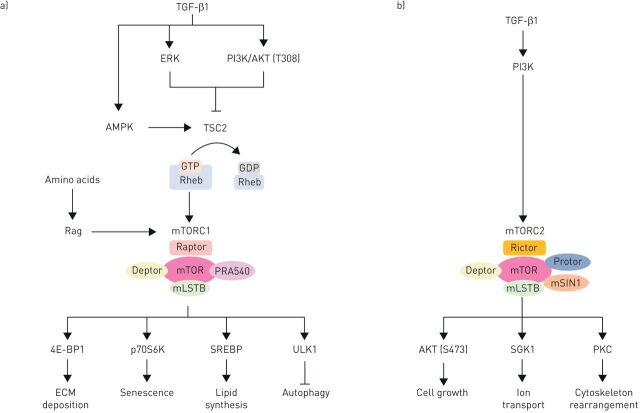
FIGURE 1.
Simplified schematic diagram of the activators and substrates of mammalian target of rapamycin complexes (mTORC)1 and mTORC2 in the context of fibrosis. a) mTORC1 activity is highly dependent on RAS homologue enriched in brain (Rheb), which is under negative regulatory control by tuberous sclerosis complex (TSC)1/2. TSC1/2 converts RhebGTP into RhebGDP. For mTORC1 to become activated through this axis, TSC1/2 must therefore be inhibited. The phosphorylation of different sites within the TSC1/2 complex in response to extracellular signal-regulated kinaes (ERK) and phosphoinositide-3-kinase (PI3K)/protein kinase B (AKT) pathways leads to activation of mTORC1; whereas activation of 5' AMP-activated protein kinase (AMPK) suppresses mTORC1. Transforming growth factor (TGF)-β1 can activate PI3K/AKT, ERK and AMPK (through TAK1), however, the inhibition of neither of those kinases is able to modulate TGFβ1-mediated mTORC1 activity. In the presence of amino acids, the Rags can release TSC1/2 from the lysosome and instead recruit mTORC1 to bring it into close contact with Rheb. Upon activation, mTORC1 phosphorylates its substrates Eukaryotic Translation Initiation Factor 4E Binding Protein 1 (4E-BP1), ribosomal protein S6 kinase beta-1 (p70S6K), sterol regulatory element-binding protein (SREBP) and Unc-51 Like Autophagy Activating Kinase 1 (ULK1) which in turn regulate extracellular matrix (ECM) deposition, senescence, lipid synthesis and autophagy. b) mTORC2 activity is highly dependent on PI3K activity. Upon activation, mTORC2 phosphorylates AKT, SGK1 and protein kinase C (PKC) to control cell growth, ion transport and cytoskeleton rearrangement.