Abstract
The extracellular matrix (ECM) is the scaffold that provides structure and support to all organs, including the lung; however, it is also much more than this. The ECM provides biochemical and biomechanical cues to cells that reside or transit through this micro-environment, instructing their responses. The ECM structure and composition changes in chronic lung diseases; how such changes impact disease pathogenesis is not as well understood. Cells bind to the ECM through surface receptors, of which the integrin family is one of the most widely recognised. The signals that cells receive from the ECM regulate their attachment, proliferation, differentiation, inflammatory secretory profile and survival. There is extensive evidence documenting changes in the composition and amount of ECM in diseased lung tissues. However, changes in the topographical arrangement, organisation of the structural fibres and stiffness (or viscoelasticity) of the matrix in which cells are embedded have an undervalued but strong impact on cell phenotype. The ECM in diseased lungs also changes in physical and biomechanical ways that drive cellular responses. The characteristics of these environments alter cell behaviour and potentially orchestrate perpetuation of lung diseases. Future therapies should target ECM remodelling as much as the underlying culprit cells.
Short abstract
Important biochemical and biomechanical cues from the extracellular matrix are disrupted in chronic lung diseases, altering the fate of resident lung cells and trafficking immune cells, and thereby contributing to disease development and progression https://bit.ly/3FgiQvc
Introduction
The most abundant protein in our body is collagen. Its name originates from the Latin noun colle, which means glue. This describes one of the most important functions of collagen and, for that matter, of other extracellular matrix (ECM) constituents: these form the tissue glue that keeps the cells in tissues and organs together. The scaffolding function of the ECM provides architecture and strength, and was long considered its main, if not only, function [1]. This misconception has been acknowledged with improved understanding of the composition of the ECM. In a reductionist's view, the ECM comprises proteins, the majority of which are fibrous, embedded in a water-retaining hydrogel of highly negatively charged polydisaccharides called glycosaminoglycans (GAGs) (figure 1). Many of these GAGs are organised in higher-order structures through binding to core proteins to form proteoglycans. Proteins also form intermolecular connections, via adaptor proteins, by direct interactions or through covalent cross-linking. The ECM is dynamic, which means it is continuously remodelled by the embedded cells that respond to micro-environmental biochemical and mechanical cues. Cell-secreted soluble signalling factors such as growth factors and immune factors often harbour heparin-binding sites that facilitate their retention by GAGs. Upon tissue damage these factors are immediately available to initiate wound healing. Obviously, this is a classic chicken or egg paradox: does the ECM influence cells or is it the other way around? In this review, we advocate that it is both that have important consequences for lung diseases.
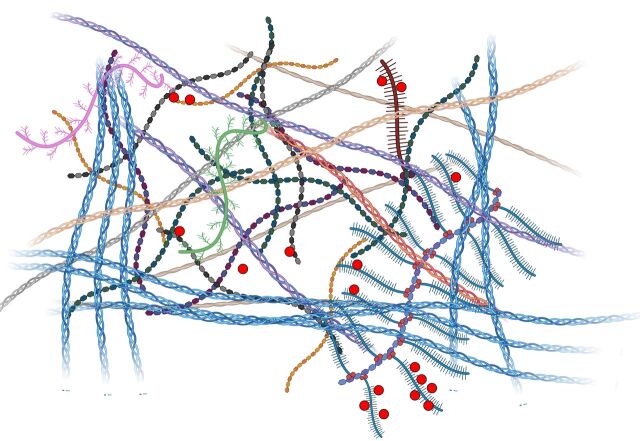
FIGURE 1.
The basic architecture of the extracellular matrix comprises fibrillar constituents (helices) that are embedded in a hydrogel of glycosaminoglycans (hexagonal repeat units) and proteoglycans (brushed molecules) with bound signalling factors (red circles). Figure partially created using BioRender.com.
ECM biochemical composition and cellular interactions
Tissues and organs vary in function, and so does their ECM composition. For example, collagen II is only present in our natural shock absorber, cartilage. On the other hand, elastin is present in many mechanically active organs that demand elasticity, although it is enriched in arterial walls and the lungs. Throughout the animal kingdom, the sequences of ECM constituents are evolutionarily highly conserved [2]. In fact, collagen I is considered one of the oldest molecules of life, which shows that the ECM and connective tissue are pivotal for multicellular organisms [3]. The ECM provides not only architectural and physical support to embedded cells but also instructs cells biochemically.
The major constituent of the ECM is collagen, a triple-helical protein with intermolecular cross-links. The collagen superfamily has nearly 30 members, which comprise fibrillar types that are the scaffolding of the ECM's architecture, network-forming collagens such as collagen IV in the basement membrane, membrane collagens and fibril-associated collagens (FACITs) that bridge ECM molecules [4]. Fibronectin is another indispensable ECM component present as dimers. This large molecule (∼500-kDa monomer) is secreted by fibroblasts as an insoluble fibre meshwork that paves the way for further matrix construction. Cells bind with their ECM-specific surface receptors, e.g. integrins α5β1, α3β1 and αvβ3, to fibronectin [5]. The best-studied integrin-binding motif in fibronectin is the tripeptide Arg-Gly-Asp (RGD). However, fibronectin also binds to collagens and to GAGs, and thus literally connects and bridges the ECM network (figure 2).
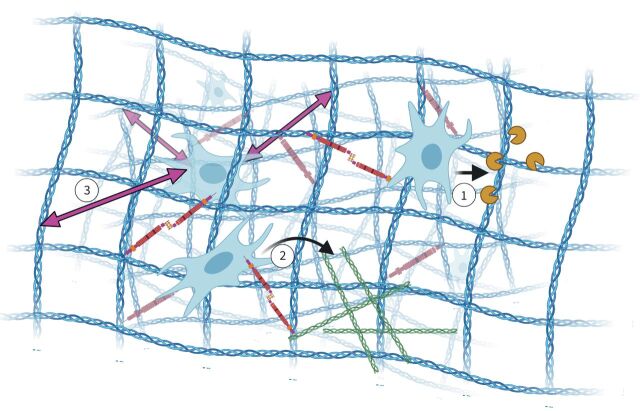
FIGURE 2.
Extracellular matrix (ECM) architecture and remodelling. Cells reside in a fibrous network of collagens (blue) to which they bind, as well as to fibronectin (brown) that also binds to collagen. During maintenance and remodelling, 1) connective tissue cells secrete proteases (dark yellow “pacmans”) that degrade the collagen network (blue). 2) Next, these cells deposit new ECM components such as collagens (green), and 3) in this way the architecture is maintained and forces (purple double-headed arrows) are transmitted to the embedded cells. Figure partially created using BioRender.com.
The fibrous network of ECM proteins is embedded in a water-retaining hydrogel of the negatively charged GAGs. The majority are sulfated GAGs (sGAGs) such as heparan sulfate, chondroitin sulfate, aggrecan and dermatan sulfate; hyaluronic acid is the only GAG that has acidic side chains. (s)GAGs are usually assembled as higher-order structures through binding via adaptor proteins to a protein core to yield proteoglycans, of which heparan sulfate proteoglycan is a prime member [6]. The (s)GAGs and proteoglycans bind a plethora of growth factors and chemokines, and store these for “release on demand” (e.g. after tissue damage) [7]. Glycobiology has proven a challenging research field due the lack of adequate (high-throughput) analysis methods, as well as the high complexity of GAGs. This complexity is several orders higher than that of the most well-known polysaccharide, i.e. DNA.
The ECM contains a number of extracellular modulators of cell function [8]. These matricellular proteins regulate cell adhesion and migration through bridging cells with ECM structural components [9, 10].
All ECM constituents are prone to enzymatic hydrolysis, which also leads to a host of small degradation products with biological activity called matrikines and matricryptins [11]. These short-lived regulators influence tissue repair and fibrosis, often in a beneficial way [12]. An example is endostatin, the 20-kDa C-terminal fragment of collagen XVIII that inhibits angiogenesis [13]. In lungs, the combined action of matrix metalloproteinases (MMPs) and prolylendopeptidase may release the tripeptide matrikine Pro-Gly-Pro (PGP) from collagens. In its pristine or acetylated form, PGP regulates repair. The persistent presence of PGP attracts and activates neutrophils, which contributes to lung pathology [14].
Finally, the ECM contains matricellular proteins such as thrombospodin, tenascin, osteopontin and fibulins, which as the name suggests influence cellular function literally from the matrix side [15, 16]. These, often large, matricellular proteins have integrin-binding sites, as well ECM-binding sites, yet also bind growth factors. As an example, fibulins bind to collagens, fibronectin and elastin to assist in fibre formation, but also harbour integrin-binding sites for cells [17, 18].
The ECM is continuously remodelled, which involves specific matrix-directed proteases such as MMPs (figure 2). Of the 28 MMPs, most degrade collagens and are inhibited by tissue inhibitors of MMPs (TIMPs) [19].
The cell surface is littered with receptors that bind to the ECM. The heterodimeric αβ-integrins are most abundant. β1-integrin (CD29) is present on virtually every cell in the body. Integrins bind to short amino acid stretches, e.g. RGD (fibronectin binding) and GROGER (collagen binding) [20]. Other connections to the ECM are hemidesmosomes in which α4β6-integrin binds to laminin in the basement membrane, but it depends on binding to collagen XVII (BP180) to maintain the hemidesmosome structure [21]. Most cells adhere to a basal membrane. This very dense and rather thin layer (∼25 nm) of ECM consists of two nonfibrous proteins (collagen IV and laminin) and two proteoglycans (nidogen and perlecan) that mutually interact [22]. The lung basement membrane is altered during lung disease [23], such as pulmonary arterial hypertension [24].
Cells also express specific collagen-binding receptors such as discoidin domain receptor 1 and 2 that recognise the amino acid sequence GVMGFO. Others such as Endo180, glycoprotein VI and LAIR recognise Gly-Pro-Hyp amino acid stretches in collagen [20]. Interestingly, CD44 binds to collagen but is mainly recognised as the surface receptor for the GAG hyaluronic acid. In turn, hyaluronic acid is also recognised by Toll-like receptors (TLRs). CD44 also binds to collagens, matricellular proteins such as osteopontin and matrix-degrading enzymes such as MMPs. This shows a strong redundancy in the interactions between cellular receptors and ECM constituents. All of these ECM receptors have relatively small intracellular domains that connect to complexes of adaptor molecules for facilitating signal transduction.
The mechanical nature of the lung demands the lung ECM to adapt accordingly: flexible yet strong and with defined architectures to facilitate transport of air (airways) and gas exchange (alveoli). The connective tissue continuously maintains these different structures. Connective tissue comprises vasculature and mesenchymal cells that are embedded in the ECM. The predominant mesenchymal cell is the fibroblast that, as the name implies, is responsible for the production of fibres primarily comprising collagens. Essentially, the fibroblast is a professional ECM remodeller that senses its environment in terms of biochemistry and physical stimuli, and responds accordingly. Fibroblasts are surrounded by the ECM and seldom organise as closely connected cells. In contrast, lung epithelial cells and endothelial cells are polarised and attach to a basement membrane at their basal side; the apical side being exposed to air or blood, respectively. The continuous remodelling of the ECM is a process of degradation, orchestrated by matrix-specific proteases and hydrolases, and simultaneous deposition and processing of novel ECM constituents and their cross-linking by specific enzymes such as the lysyl oxidase (LOX) family of enzymes.
Environmental triggers such as tissue damage or environmental insults change the fibroblast phenotype towards tissue repair. This is accompanied by degradation of the ECM to literally make space for repair cells such as immune cells and ingrowing vessels. During the resolution phase of wound repair, the ECM is restored. The fibroblasts differentiate to myofibroblasts during this process. These are contractile, proliferative and motile cells that deposit more ECM than the average maintenance fibroblast. Normal wound healing resolves through apoptosis of myofibroblasts, yet in pathological wound healing these acquire apoptosis resistance. In fact, matrix stiffness itself may render myofibroblasts in an anti-apoptotic state (reviewed in [25]).
While this repair process is generally well regulated during repair of physiological damage, chronic insults of the lung such as smoking, inhalation of airborne deleterious particles or organic solvents negatively affect the repair process. The result may either be a drastic degradation of lung parenchyma such as in emphysema, or it may manifest as a pathological increase in ECM deposition such as in asthma, where the airways are chronically inflamed and thickened due to excess ECM, or in fibrotic lung disease, with idiopathic pulmonary fibrosis (IPF) being a key example, where there is excessive scarring of lung tissue by continuous deposition of heavily cross-linked ECM.
ECM composition changes in chronic lung pathologies
It is well recognised that the composition of the ECM in the lung changes during the pathological processes that underlie chronic lung diseases. While capturing the exact nature of these changes has been challenging, with different studies difficult to compare owing to technical variability in the region of tissue examined, the fixation and preservation variances, and limited tools, the emerging catalogue of changes in ECM components in asthma, chronic obstructive pulmonary disease (COPD) and fibrotic lung diseases has been summarised in several recent reviews [26–29].
It now evident that the ECM composition that mesenchymal cells from the lung encounter is a major factor in determining their responses. Parker et al. [30] elegantly illustrated that the origin of the ECM scaffold in which cells were seeded, rather than the source of the cells themselves, was the determining factor in the resultant measured gene expression profiles. The decellularised scaffolds derived from lungs from donors with IPF induced increased gene transcriptional profiles when compared with scaffolds from healthy donors. We have previously reported that airway smooth muscle cells exposed to an ECM deposited by cells derived from donors with asthma have a faster proliferative rate than those on an ECM derived from cells from nonasthmatic donors [31]. When the C isoform of the matricellular protein fibulin-1 is not incorporated in these matrix beds, the enhanced proliferative induction from the asthmatic ECM is no longer evident. Similarly, mice that lack the fibulin-1C isoform are protected from excessive ECM deposition when exposed to stimuli that model asthma (ovalbumin or house dust mite), COPD (cigarette smoke) or IPF (bleomycin) [32–34]. Intriguingly, we have shown that the lack of fibulin-1C in the ECM reduces the enhanced activity of transforming growth factor (TGF)-β via an interaction with latent TGF-binding protein 1 [32]. These data illustrate the importance of the composition of the ECM in directing cellular responses. Changes in the components of the ECM, as is evident during lung disease pathogenesis, are likely to contribute to the reprogramming of cells that then drive the ongoing disease pathogenesis.
Turnover of some constituents of the ECM is a very dynamic process. Early studies in rat lungs suggest that the turnover of the collagen matrix of the lungs is 10–15% per day [35]. Other ECM proteins are recognised to be much more stable, with suggestions that the elastin elements in the lung tissue are incorporated during development and cannot be renewed in adult life. This is one of the major challenges when the elastin fibres are disrupted in lung diseases such as emphysema; restoring these essential elements of the lung ECM is a challenge for regenerative medicine. One of the often overlooked results of the dynamic turnover of the ECM is the release of soluble degradation products. The altered composition of the ECM in chronic lung disease is also evident in the profile of degradation products that can be measured in serum, plasma or bronchoalveolar lavage from patients. ECM fragments, the matricryptins or matrikines, particularly fragments from collagen molecules, are emerging as potential markers for active disease processes or indicators of likelihood of progression in lung diseases.
In the PROFILE study, higher levels of neoepitopes derived from the degradation of collagen I (C1M), collagen III (C3M) and collagen VI (C6M) were observed in serum at baseline in patients with IPF compared with healthy controls [36]. The levels of C1M and C3A (the fragment generated following collagen III degradation by ADAMTS-1/4/8) at baseline were associated with mortality in this patient group, while high levels of the markers PRO-C3 and PRO-C6 (neoepitopes indicative of the production of newly formed collagen III and collagen VI, respectively) in IPF patients were indicative of likelihood of disease progression [37]. Similarly, in the COPDGene study, C6M, PRO-C6 and EL-NE (the elastin fragment released following degradation by neutrophil elastase) were increased in COPD patients’ serum compared with never-smoking controls. In COPD patients the levels of C1M, PRO-C6 and EL-NE were inversely related to lung function (forced expiratory volume in 1 s) [38]. Similar observations were made in the BASCO and PROMISE-COPD cohorts where the serum levels of PRO-C3, PRO-C6, C3M, C6M and EL-NE were related to lung function. PRO-C3 levels were associated with a greater likelihood of exacerbation and PRO-C6 levels with survival [39]. In the ECLIPSE COPD cohort, C4Ma3 (the degradation marker from collagen IV) was found to be higher in COPD patients who were at higher risk of mortality, with the levels being related to the number of hospitalisations due to exacerbations [40].
The released ECM fragments should also be considered active elements in cell–matrix interactions. These fragments can interact with many cell surface receptors, including integrins, TLRs, growth factor receptors and other diverse receptors, to induce cellular responses. These responses often vary from those induced by the parent ECM molecule. ECM fragments can also influence the proteolytic activity in the lung through their direct actions as enzymes or as inhibitors of enzyme activity, or they can regulate the process of pro-enzyme activation.
Highlights: ECM composition
1) The ECM composition changes in chronic lung diseases; the ratio of components becomes distorted. 2) There is increased ECM deposition during fibrosis, but degradation in emphysema. 3) Unique fragments released from the ECM can indicate active disease processes, but also contribute to cell responses to the ECM.
ECM topography and organisation
Impact of ECM topography and organisation in terms of cell interactions with the ECM
The surface topography of the ECM is sensed by adhered cells. For lower-order structures (molecules) at the nanoscale, this includes the thickness and surface roughness or smoothness of fibre structures. At a higher organisation level, such as fibrous networks (microscale), parameters that determine topography include bundle size and thickness, protrusions, and patterns of grates, pillars and grooves/ridges, while flat surfaces are rare (figure 3). Nanoscale topography, e.g. of basement membranes but also single collagen or fibronectin molecules, has already been studied in the second half of the last century with the electron microscope [41]. Nanoscale topography such as surface roughness influences proliferation, adhesion and morphology, e.g. of cultured fibroblasts [42]. Linearly aligned collagen fibres induce unidirectional migration of fibroblasts [43]. While microscale topography is challenging to assess in tissue or hydrogels, extensive knowledge about cellular behaviour has been gained using artificially generated topographies on silicone rubber (dimethylpolysiloxane), as well as on other natural and synthetic biomaterials (reviewed in [44, 45]). Whether cells stay on top of the topography or whether cells engulf the topography depends on the hydrophobicity and adsorbed proteins [46, 47]. Engulfing the topography alters the membrane curvature of the cells, which induces topotaxis [48, 49]. The topography exerts its effects predominantly through ECM–integrin-mediated activation of focal adhesion kinase (FAK) complexes [48, 50].
FIGURE 3.
Topography examples: a) flat surface, b) pillars, and c) linear grooves and ridges.
ECM topography and organisation changes in chronic lung disease
Evidence is emerging that illustrates that the structural landscape within the ECM of a diseased lung is very different to that in a healthy lung. Second harmonic generation imaging illuminates the differences in collagen organisation in tissues or cell-derived matrices from donors with asthma [51], COPD [52, 53] and IPF [54]. Collagen fibre assembly and cross-linking impact the organisational structure that dominates the lung architecture. The regulation of this process is tightly controlled under homeostatic conditions, at many levels both intracellularly and extracellularly [55]. Many classes of enzymes contribute to these regulatory controls, of which the transglutaminase and LOX families have been a focus of attention in chronic lung diseases. In both COPD and IPF, dysregulation of transglutaminase 2 and LOX-like 1 and 2 have been reported in cells and tissues, with the direction of these changes varying between the disease states (decreases in COPD and increases in IPF) [54, 56–59]. The differences in levels of expression and/or activity of these collagen cross-linking regulatory enzymes in the different disease states may provide keys to understanding some of the mechanisms underlying the altered ECM topography and organisation in lung diseases. Targeting matrix stiffness and mechanical signalling is a promising future therapeutic prospect, e.g. via LOXs [60, 61]. Importantly, LOXs have been reported to be essential for cells to actively remodel their micro-environment, with lung fibroblasts actively remodelling collagen fibres in their immediate vicinity after exposure to TGF-β, a process that is inhibited in the presence of the pan-LOX inhibitor 3-aminopropionitrile (β-aminopropionitrile (BAPN)) [54].
Contribution of ECM topography and organisation to lung disease pathophysiology
The question then arises as to what are the impacts of these changes on ECM topography and organisation? From in vitro cell models we learn that the ECM deposited by fibroblasts from donors with IPF differs topographically from that of cells from donors without disease. The IPF-derived ECM induces increased cell adhesion and proliferation; however, if those same ECMs are deposited in the presence of the LOX inhibitor BAPN then these differences are abrogated [62]. Similar differences in structural arrangement are also observed in decellularised scaffolds from fibrotic (IPF) versus control lung tissues [63]. Reseeding cells of mesenchymal origin in such scaffolds reveals that the fibrotic scaffolds, with their aberrant topography and organisation, induce increased gene transcription [30] and also the release of extracellular vesicles with pro-fibrotic microRNAs [64].
Immune cells also respond to the topography of ECM fibres that they encounter. For such cells the ECM fibres are the highways along which they traffic within the tissues. Once again, when the structural arrangement of the ECM fibres changes, so do the immune cell responses. When macrophages encounter fibrous collagen fibres they take on a rounded morphology with dense actin filaments at their cell membrane. Vasse et al. [65] found that these macrophages had a faster migratory rate than those on globular collagen fibres. In contrast, the macrophages on the globular collagen had actin filaments throughout the cells and had distinct filopodia. The authors suggested these different collagen environments were inducing different modes of migration, with an amoeboid manner being active on the fibrous surface, while a more mesenchymal migration was enacted on the globular collagen [65]. While not explored in the lung as yet, Nicolas-Boluda et al. [66] recently reported that altering ECM cross-linking in a tumour micro-environment dictated the migratory patterns of T-cells. When remodelling of the collagen network was inhibited through the use of BAPN, and thus collagen cross-linking was prevented, the migration of T-cells into the tumour environment was enhanced in mouse models of tumour development [66].
Highlights: ECM topography and organisation
1) ECM topography changes in chronic lung diseases; disrupted topography changes cell interactions. 2) ECM organisation is regulated by cross-linking enzymes, the levels of which are dysregulated in lung diseases. 3) Immune cell trafficking is regulated by ECM topography and organisation.
ECM biomechanics
Impact of ECM biomechanics in terms of cell interactions with the ECM
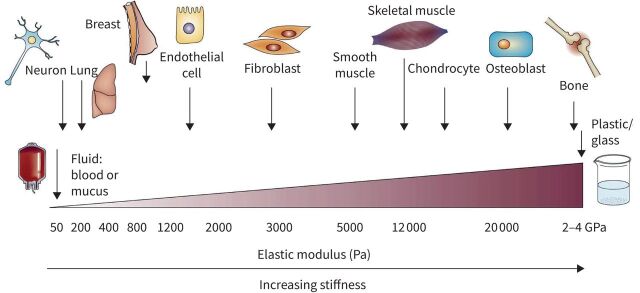
The ECM exerts forces that are sensed and responded to by cells [67]. A static mechanical component of the ECM is its stiffness or elastic modulus (an object's resistance to deformation). Tissues vary in stiffness between tens/hundreds of pascals (e.g. lung) to gigapascals (e.g. bone) (figure 4) [68, 69].
FIGURE 4.
Stiffness (elastic modulus) of cells and tissues. Reproduced from [68] with permission.
Engler and co-workers [70, 71] showed that cellular plasticity such as myoblast or mesenchymal stem cell differentiation is not only dictated by topography but also relies on natural tissue stiffness. Other forces exerted on cells include pulling, compression, tearing, shearing and twisting, and might be of a cyclic nature such as in the continuously inflating and deflating lungs. Mechanosensing and its transduction to the cytoplasm and nucleus relies on cell surface integrins bound to ECM molecules. Integrins cluster and form focal adhesions on the cytoplasmic side of the cell membrane, which are connected to the cortical actin cytoskeleton and transduce mechanical signals [72]. In addition, the FAK and integrin-linked kinase in focal adhesions upregulate small GTPases such as RhoA and ROCK. Ultimately, this promotes various processes, mainly including contraction, migration, survival and proliferation. The hyaluronic acid receptor CD44 also “mechanosignals” through release of its intracellular domain (CD44ICD). Almost three decades ago, Ingber [73–75] coined the term “tensegrity” (tension integration) to describe the information-conducting mechanism through which deformation of cells causes disruption of the self-supporting cortical actin cytoskeleton that is relayed throughout the cell and contributes to mechanosignalling. The actin cytoskeleton also physically interacts with the nuclear lamina, and in this way directly changes the chromatin structure and thus gene expression [76, 77]. The tensegrity of cyclically stretched substrates (lungs) has been mathematically modelled [78]. Tensegrity works both ways: cells also mechanically influence the local stiffness of the surrounding ECM [79]. Knowledge of tensegrity was indispensable for the development of the “human-airway-on-a-chip” [80]. Currently, much of the topographical and biomechanical knowledge about the ECM is being translated into novel regenerative medicine-based approaches to treat disease in the future [81]. Of note, we showed that the stiffness of cultured fibroblasts from IPF patients was higher than that of nondiseased control fibroblasts [82]. Moreover, this likely correlates with the much higher stiffness of fibrotic lung ECM (19 kPa) compared with healthy lung ECM (4 kPa) [83].
How does ECM biomechanics contribute to lung disease pathophysiology?
The biomechanical environment within the lung tissue is the result of the complex mix of ECM components, the cross-linking of these components and the surfactant system that lines the alveolar epithelial surfaces [84]. Disruption of lung mechanics that results from surfactant system genetic disorders will not be covered in this review, rather the reader is referred to Van Moorsel et al. [85]. The gas exchange units, the alveoli, are kept open through transpulmonary pressure from the air in the lungs. This pressure, referred to as pre-stress, is counterbalanced by the forces exerted from within the tissue and the surfactant film surface forces, with the main pathway for the transmission of this stress being through the ECM [86]. Therefore, the mechanical properties of the tissue (ECM), both at a macroscopic and a microscopic level, have key roles in determining the lung function and cellular responses within these tissues. In turn, the mechanical properties of the tissue are dictated by the major components within the ECM, in particular collagens, elastin and proteoglycans [87].
Once we recognise that the constituents of the lung tissue are important for underpinning the mechanical properties, we can begin to understand the altered biomechanical environment that exists in diseased lung tissues. In IPF the lung tissue is stiffer than in control tissues [63] and this altered stiffness is maintained even after these tissues are decellularised. With COPD the biomechanical profile is a little more complicated. It is recognised that the ECM that surrounds the small airways becomes stiffer, which has immediate consequences for airway hyperresponsiveness [29]. The overall parenchymal tissue in COPD appears softer, mainly owing to the enlarged airspaces in the emphysematous regions. However, the remaining alveolar walls, which contain at least as much collagen as detected in the alveolar walls in control lung tissue, have similar stiffness profiles to that of healthy lung tissue [88], although their susceptibility to rupture is greater. We recently reported that these stiffness profiles of the different lung tissues are reflected in the mechanical properties of hydrogels generated from decellularised ECM from IPF, COPD or control lung tissues, again reflecting how the constituents of the ECM are essential for driving the mechanical properties [83].
The stiffness of the ECM is a major determinant for functional responses of cells within this environment [89]. Jamieson et al. [90] recently illustrated that the stiffness of collagen fibres governed the propensity of an airway to contract in the presence of a stimulus. Cross-linking of collagen fibres, reflecting the remodelled ECM found in an asthmatic airway, resulted in a stiffer airway wall that constricted to a greater degree and at a faster rate. Similarly, Maarsingh et al. [91] showed, using precision cut lung slices from COPD and non-COPD donors, that small airway hyperresponsiveness reflected the altered ECM mechanical properties in COPD, with exaggerated stiffness differences of the ECM surrounding the airway being central in controlling these responses.
The high stiffness of the ECM in an IPF tissue is a typical example of how the ECM negatively instructs cells via mechanosignalling (figure 5). If pulmonary fibroblasts isolated from healthy lungs are cultured on ECM derived from IPF lung, these adopt a pro-fibrotic phenotype (figure 5) [30]. When the fibroblasts are exposed to substrates of increasing stiffness, ranging from that of normal lung physiological stiffness up to the gigapascal range of tissue culture plastic, an advancing profile of increasing adhesion rate, proliferation and migratory capacity is observed [92]. Such findings are reflective of knowledge generated in the tumour literature, where the stiffer ECM environment is advantageous for tumour growth [66]. The cross-linking enzymes that regulate the stiffness of the collagen fibres are essential in the regulation of these micro-environmental changes, as illustrated by the blockade of LOX family member activity resulting in reductions in stiffness in model systems [54, 66, 93]. Dysregulation of these and other cross-linking enzymes in chronic lung diseases may be instrumental in contributing to the altered stiffness in these tissues. While a clinical trial in 2017 targeting LOX-like 2 in IPF was stopped early due to a lack of efficacy [94], this family of enzymes continues to receive attention as potential vehicles for modulation of tissue stiffness in fibrosis [93, 95]. Further research in this area is warranted to understand the mechanisms that lead to increased tissue stiffness and possible ways to alleviate these changes in chronic lung diseases.
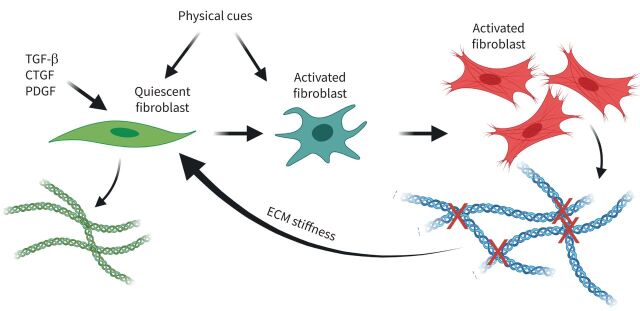
FIGURE 5.
Self-propelling, stiff extracellular matrix (ECM)-induced lung fibrosis. Initially, growth factor-activated fibroblasts (dark green) progress to fully activated fibroblasts (red) that increase stiffness through excessive collagen cross-linking, which provides physical cues to activate fibroblasts. TGF-β: transforming growth factor-β; CTGF: connective tissue growth factor; PDGF: platelet-derived growth factor. Figure partially created using BioRender.com.
Highlights: ECM stiffness
1) The mechanical properties of the tissue, both at a macroscopic and a microscopic level, have key roles in determining the lung function and cellular responses within these tissues. 2) IPF tissues have increased stiffness compared with control tissues. 3) Micro-environmental stiffness regulates cell adhesion, migration and proliferation. 4) ECM cross-linking enzymes, e.g. LOX family enzymes, are crucial for regulating stiffness.
Discussion
Through its multiple biochemical and biomechanical elements of influence, the ECM constitutes a bioactive functional parameter that enables multicellularity and multifunctionality in our organs [96]. The interconnectivity of the ECM elements discussed in this review illustrates the complex role of the ECM in maintaining tissue homeostasis or directing disease development and/or progression (figure 6). In the years ahead the challenge will be to separate the individual contributions of each element to the processes that drive lung diseases in vivo.
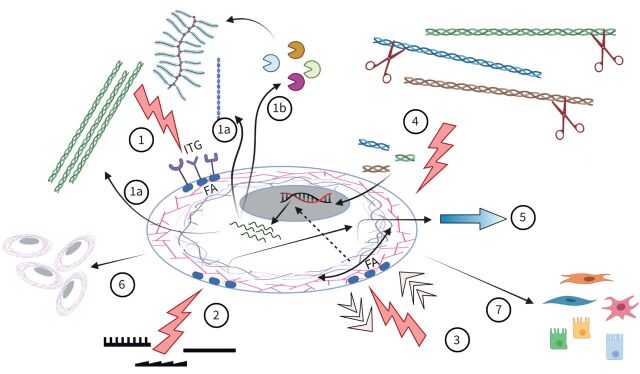
FIGURE 6.
Extracellular matrix (ECM) molecules influence cell behaviour via integrin (ITG) binding and activation of focal adhesions (FAs) (1). Mechanically, cells are influenced by topography (2) and forces (pulling/compression) (3). In response, the ECM is turned over (1a, 1b), while degradation products (matrikines) also regulate cells (4). The combined result varies from migration (5), proliferation and survival (6) to phenotypic changes (7).
The conundrum remains as to which is the chicken and which is the egg when we think about ECM changes and disease processes in the lung. In asthma the remodelling is evident in very young children well before asthma symptoms are evident [97, 98], while population-based health screening studies illustrate interstitial lung abnormalities (structure changes), as detected by high-resolution computed tomography, in the lungs well before lung function impairment is evident [99]. Just as the ECM is a driving force in developmental processes, it is now becoming clear that the ECM also drives development of disease. However, it is not too late to stop the chicken from flying the coop. The evidence presented in this review indicates that such ECM changes will alter cell behaviours, mechanistically adding to the disease perpetuation in lung diseases, thereby highlighting that future therapies should target ECM remodelling as much as the underlying culprit cells.
Footnotes
Provenance: Commissioned article, peer reviewed.
Conflict of interest: J.K. Burgess reports receiving unrestricted research funds paid to the department from Boehringer Ingelheim, outside the submitted work.
Conflict of interest: M.C. Harmsen has nothing to disclose.
References
- 1.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010; 123: 4195–4200. doi: 10.1242/jcs.023820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Har-el R, Tanzer ML. Extracellular matrix. 3: evolution of the extracellular matrix in invertebrates. FASEB J 1993; 7: 1115–1123. doi: 10.1096/fasebj.7.12.8375610 [DOI] [PubMed] [Google Scholar]
- 3.Mathews MB. Macromolecular evolution of connective tissue. Biol Rev Camb Philos Soc 1967; 42: 499–551. doi: 10.1111/j.1469-185X.1967.tb01528.x [DOI] [PubMed] [Google Scholar]
- 4.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol 2011; 3: a004978. doi: 10.1101/cshperspect.a004978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson S, Svineng G, Wennerberg K, et al. . Fibronectin–integrin interactions. Front Biosci 1997; 2: d126–d146. doi: 10.2741/A178 [DOI] [PubMed] [Google Scholar]
- 6.Bulow HE, Hobert O. The molecular diversity of glycosaminoglycans shapes animal development. Annu Rev Cell Dev Biol 2006; 22: 375–407. doi: 10.1146/annurev.cellbio.22.010605.093433 [DOI] [PubMed] [Google Scholar]
- 7.Shi D, Sheng A, Chi L. Glycosaminoglycan-protein interactions and their roles in human disease. Front Mol Biosci 2021; 8: 639666. doi: 10.3389/fmolb.2021.639666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 2002; 14: 608–616. doi: 10.1016/S0955-0674(02)00361-7 [DOI] [PubMed] [Google Scholar]
- 9.Mosher DF, Adams JC. Adhesion-modulating/matricellular ECM protein families: a structural, functional and evolutionary appraisal. Matrix Biol 2012; 31: 155–161. doi: 10.1016/j.matbio.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 10.Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol 2014; 37: 1–14. doi: 10.1016/j.matbio.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricard-Blum S, Salza R. Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Exp Dermatol 2014; 23: 457–463. doi: 10.1111/exd.12435 [DOI] [PubMed] [Google Scholar]
- 12.de Castro Bras LE, Frangogiannis NG. Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol 2020; 91–92: 176–187. doi: 10.1016/j.matbio.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Reilly MS, Boehm T, Shing Y, et al. . Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997; 88: 277–285. doi: 10.1016/S0092-8674(00)81848-6 [DOI] [PubMed] [Google Scholar]
- 14.Patel DF, Snelgrove RJ. The multifaceted roles of the matrikine Pro-Gly-Pro in pulmonary health and disease. Eur Respir Rev 2018; 27: 180017. doi: 10.1183/16000617.0017-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal 2009; 3: 163–165. doi: 10.1007/s12079-009-0069-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerarduzzi C, Hartmann U, Leask A, et al. . The matrix revolution: matricellular proteins and restructuring of the cancer microenvironment. Cancer Res 2020; 80: 2705–2717. doi: 10.1158/0008-5472.CAN-18-2098 [DOI] [PubMed] [Google Scholar]
- 17.Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol 2014; 802: 31–47. doi: 10.1007/978-94-007-7893-1_3 [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T. Roles of short fibulins, a family of matricellular proteins, in lung matrix assembly and disease. Matrix Biol 2018; 73: 21–33. doi: 10.1016/j.matbio.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 19.Raeeszadeh-Sarmazdeh M, Do LD, Hritz BG. Metalloproteinases and their inhibitors: potential for the development of new therapeutics. Cells 2020; 9: 1313. doi: 10.3390/cells9051313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol 2007; 26: 146–155. doi: 10.1016/j.matbio.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 21.Hopkinson SB, Findlay K, deHart GW, et al. . Interaction of BP180 (type XVII collagen) and alpha6 integrin is necessary for stabilization of hemidesmosome structure. J Invest Dermatol 1998; 111: 1015–1022. doi: 10.1046/j.1523-1747.1998.00452.x [DOI] [PubMed] [Google Scholar]
- 22.Kruegel J, Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cell Mol Life Sci 2010; 67: 2879–2895. doi: 10.1007/s00018-010-0367-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchiya T, Doi R, Obata T, et al. . Lung microvascular niche, repair, and engineering. Front Bioeng Biotechnol 2020; 8: 105. doi: 10.3389/fbioe.2020.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambade AS, Hassoun PM, Damico RL. Basement membrane extracellular matrix proteins in pulmonary vascular and right ventricular remodeling in pulmonary hypertension. Am J Respir Cell Mol Biol 2021; 65: 245–258. doi: 10.1165/rcmb.2021-0091TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinz B, Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol 2020; 16: 11–31. doi: 10.1038/s41584-019-0324-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess JK, Mauad T, Tjin G, et al. . The extracellular matrix – the under-recognized element in lung disease? J Pathol 2016; 240: 397–409. doi: 10.1002/path.4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgstaller G, Oehrle B, Gerckens M, et al. . The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J 2017; 50: 1601805. doi: 10.1183/13993003.01805-2016 [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Philp AM, Corte T, et al. . Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol Ther 2021; 225: 107839. doi: 10.1016/j.pharmthera.2021.107839 [DOI] [PubMed] [Google Scholar]
- 29.Bidan CM, Veldsink AC, Meurs H, et al. . Airway and extracellular matrix mechanics in COPD. Front Physiol 2015; 6: 346. doi: 10.3389/fphys.2015.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker MW, Rossi D, Peterson M, et al. . Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 2014; 124: 1622–1635. doi: 10.1172/JCI71386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau JY, Oliver BG, Baraket M, et al. . Fibulin-1 is increased in asthma – a novel mediator of airway remodeling? PLoS One 2010; 5: e13360. doi: 10.1371/journal.pone.0013360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G, Cooley MA, Jarnicki AG, et al. . Fibulin-1c regulates transforming growth factor-beta activation in pulmonary tissue fibrosis. JCI Insight 2019; 5: e124529. doi: 10.1172/jci.insight.124529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Cooley MA, Jarnicki AG, et al. . Fibulin-1 regulates the pathogenesis of tissue remodeling in respiratory diseases. JCI Insight 2016; 1: e86380. doi: 10.1172/jci.insight.86380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu G, Cooley MA, Nair PM, et al. . Airway remodelling and inflammation in asthma are dependent on the extracellular matrix protein fibulin-1c. J Pathol 2017; 243: 510–523. doi: 10.1002/path.4979 [DOI] [PubMed] [Google Scholar]
- 35.McAnulty RJ, Laurent GJ. Collagen synthesis and degradation in vivo. Evidence for rapid rates of collagen turnover with extensive degradation of newly synthesized collagen in tissues of the adult rat. Coll Relat Res 1987; 7: 93–104. doi: 10.1016/S0174-173X(87)80001-8 [DOI] [PubMed] [Google Scholar]
- 36.Jenkins RG, Simpson JK, Saini G, et al. . Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med 2015; 3: 462–472. doi: 10.1016/S2213-2600(15)00048-X [DOI] [PubMed] [Google Scholar]
- 37.Organ LA, Duggan A-MR, Oballa E, et al. . Biomarkers of collagen synthesis predict progression in the PROFILE idiopathic pulmonary fibrosis cohort. Respir Res 2019; 20: 148. doi: 10.1186/s12931-019-1118-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bihlet AR, Karsdal MA, Sand JM, et al. . Biomarkers of extracellular matrix turnover are associated with emphysema and eosinophilic-bronchitis in COPD. Respir Res 2017; 18: 22. doi: 10.1186/s12931-017-0509-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stolz D, Leeming DJ, Kristensen JHE, et al. . Systemic biomarkers of collagen and elastin turnover are associated with clinically relevant outcomes in COPD. Chest 2017; 151: 47–59. doi: 10.1016/j.chest.2016.08.1440 [DOI] [PubMed] [Google Scholar]
- 40.Ronnow SR, Sand JMB, Langholm LL, et al. . Type IV collagen turnover is predictive of mortality in COPD: a comparison to fibrinogen in a prospective analysis of the ECLIPSE cohort. Respir Res 2019; 20: 63. doi: 10.1186/s12931-019-1026-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vakonakis I, Campbell ID. Extracellular matrix: from atomic resolution to ultrastructure. Curr Opin Cell Biol 2007; 19: 578–583. doi: 10.1016/j.ceb.2007.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pennisi CP, Dolatshahi-Pirouz A, Foss M, et al. . Nanoscale topography reduces fibroblast growth, focal adhesion size and migration-related gene expression on platinum surfaces. Colloids Surf B Biointerfaces 2011; 85: 189–197. doi: 10.1016/j.colsurfb.2011.02.028 [DOI] [PubMed] [Google Scholar]
- 43.Ramirez-San Juan GR, Oakes PW, Gardel ML. Contact guidance requires spatial control of leading-edge protrusion. Mol Biol Cell 2017; 28: 1043–1053. doi: 10.1091/mbc.e16-11-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui L, Yao Y, Yim EKF. The effects of surface topography modification on hydrogel properties. APL Bioeng 2021; 5: 031509. doi: 10.1063/5.0046076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anselme K, Davidson P, Popa AM, et al. . The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater 2010; 6: 3824–3846. doi: 10.1016/j.actbio.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 46.Hansson PM, Swerin A, Schoelkopf J, et al. . Influence of surface topography on the interactions between nanostructured hydrophobic surfaces. Langmuir 2012; 28: 8026–8034. doi: 10.1021/la300628m [DOI] [PubMed] [Google Scholar]
- 47.Scopelliti PE, Borgonovo A, Indrieri M, et al. . The effect of surface nanometre-scale morphology on protein adsorption. PLoS One 2010; 5: e11862. doi: 10.1371/journal.pone.0011862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.SenGupta S, Parent CA, Bear JE. The principles of directed cell migration. Nat Rev Mol Cell Biol 2021; 22: 529–547. doi: 10.1038/s41580-021-00366-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lou HY, Zhao W, Zeng Y, et al. . The role of membrane curvature in nanoscale topography-induced intracellular signaling. Acc Chem Res 2018; 51: 1046–1053. doi: 10.1021/acs.accounts.7b00594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han J, Lin KH, Chew LY. Study on the regulation of focal adesions and cortical actin by matrix nanotopography in 3D environment. J Phys Condens Matter 2017; 29: 455101. doi: 10.1088/1361-648X/aa8d49 [DOI] [PubMed] [Google Scholar]
- 51.Mostaco-Guidolin LB, Osei ET, Ullah J, et al. . Defective fibrillar collagen organization by fibroblasts contributes to airway remodeling in asthma. Am J Respir Crit Care Med 2019; 200: 431–443. doi: 10.1164/rccm.201810-1855OC [DOI] [PubMed] [Google Scholar]
- 52.Tjin G, Xu P, Kable SH, et al. . Quantification of collagen I in airway tissues using second harmonic generation. J Biomed Opt 2014; 19: 36005. doi: 10.1117/1.JBO.19.3.036005 [DOI] [PubMed] [Google Scholar]
- 53.Abraham T, Hogg J. Extracellular matrix remodeling of lung alveolar walls in three dimensional space identified using second harmonic generation and multiphoton excitation fluorescence. J Struct Biol 2010; 171: 189–196. doi: 10.1016/j.jsb.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 54.Tjin G, White ES, Faiz A, et al. . Lysyl oxidases regulate fibrillar collagen remodelling in idiopathic pulmonary fibrosis. Dis Model Mech 2017; 10: 1301–1312. doi: 10.1242/dmm.030114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Onursal C, Dick E, Angelidis I, et al. . Collagen biosynthesis, processing, and maturation in lung ageing. Front Med 2021; 8: 593874. doi: 10.3389/fmed.2021.593874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burgstaller G, Sengupta A, Vierkotten S, et al. . Distinct niches within the extracellular matrix dictate fibroblast function in (cell free) 3D lung tissue cultures. Am J Physiol Lung Cell Mol Physiol 2018; 314: L708–L723. doi: 10.1152/ajplung.00408.2017 [DOI] [PubMed] [Google Scholar]
- 57.Merl-Pham J, Basak T, Knuppel L, et al. . Quantitative proteomic profiling of extracellular matrix and site-specific collagen post-translational modifications in an in vitro model of lung fibrosis. Matrix Biol Plus 2019; 1: 100005. doi: 10.1016/j.mbplus.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohlmeier S, Nieminen P, Gao J, et al. . Lung tissue proteomics identifies elevated transglutaminase 2 levels in stable chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2016; 310: L1155–L1165. doi: 10.1152/ajplung.00021.2016 [DOI] [PubMed] [Google Scholar]
- 59.Besiktepe N, Kayalar O, Ersen E, et al. . The copper dependent-lysyl oxidases contribute to the pathogenesis of pulmonary emphysema in chronic obstructive pulmonary disease patients. J Trace Elem Med Biol 2017; 44: 247–255. doi: 10.1016/j.jtemb.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 60.Lampi MC, Reinhart-King CA. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci Transl Med 2018; 10: eaao0475. doi: 10.1126/scitranslmed.aao0475 [DOI] [PubMed] [Google Scholar]
- 61.Tschumperlin DJ, Lagares D. Mechano-therapeutics: targeting mechanical signaling in fibrosis and tumor stroma. Pharmacol Ther 2020; 212: 107575. doi: 10.1016/j.pharmthera.2020.107575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Philp CJ, Siebeke I, Clements D, et al. . Extracellular matrix cross-linking enhances fibroblast growth and protects against matrix proteolysis in lung fibrosis. Am J Respir Cell Mol Biol 2018; 58: 594–603. doi: 10.1165/rcmb.2016-0379OC [DOI] [PubMed] [Google Scholar]
- 63.Booth AJ, Hadley R, Cornett AM, et al. . Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 2012; 186: 866–876. doi: 10.1164/rccm.201204-0754OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato S, Chong SG, Upagupta C, et al. . Fibrotic extracellular matrix induces release of extracellular vesicles with pro-fibrotic miRNA from fibrocytes. Thorax 2021; 76: 851. doi: 10.1136/thoraxjnl-2020-215962 [DOI] [PubMed] [Google Scholar]
- 65.Vasse GF, Kühn PT, Zhou Q, et al. . Collagen morphology influences macrophage shape and marker expression in vitro. J Immunol Regener Med 2018; 1: 13–20. doi: 10.1016/j.regen.2018.01.002 [DOI] [Google Scholar]
- 66.Nicolas-Boluda A, Vaquero J, Barrin S, et al. . Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. bioRxiv 2020; preprint [ 10.1101/2020.05.19.104430]. doi: 10.1101/2020.05.19.104430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 2005; 310: 1139–1143. doi: 10.1126/science.1116995 [DOI] [PubMed] [Google Scholar]
- 68.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 2009; 9: 108–122. doi: 10.1038/nrc2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 2011; 4: 165–178. doi: 10.1242/dmm.004077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engler AJ, Griffin MA, Sen S, et al. . Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 2004; 166: 877–887. doi: 10.1083/jcb.200405004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Engler AJ, Sen S, Sweeney HL, et al. . Matrix elasticity directs stem cell lineage specification. Cell 2006; 126: 677–689. doi: 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- 72.Kechagia JZ, Ivaska J, Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol 2019; 20: 457–473. doi: 10.1038/s41580-019-0134-2 [DOI] [PubMed] [Google Scholar]
- 73.Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci 1993; 104: 613–627. doi: 10.1242/jcs.104.3.613 [DOI] [PubMed] [Google Scholar]
- 74.Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci 2003; 116: 1157–1173. doi: 10.1242/jcs.00359 [DOI] [PubMed] [Google Scholar]
- 75.Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci 2003; 116: 1397–1408. doi: 10.1242/jcs.00360 [DOI] [PubMed] [Google Scholar]
- 76.Pennacchio FA, Nastaly P, Poli A, et al. . Tailoring cellular function: the contribution of the nucleus in mechanotransduction. Front Bioeng Biotechnol 2020; 8: 596746. doi: 10.3389/fbioe.2020.596746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janota CS, Calero-Cuenca FJ, Gomes ER. The role of the cell nucleus in mechanotransduction. Curr Opin Cell Biol 2020; 63: 204–211. doi: 10.1016/j.ceb.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 78.Xu GK, Li B, Feng XQ, et al. . A tensegrity model of cell reorientation on cyclically stretched substrates. Biophys J 2016; 111: 1478–1486. doi: 10.1016/j.bpj.2016.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mandriota N, Friedsam C, Jones-Molina JA, et al. . Cellular nanoscale stiffness patterns governed by intracellular forces. Nat Mater 2019; 18: 1071–1077. doi: 10.1038/s41563-019-0391-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Si L, Bai H, Rodas M, et al. . A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat Biomed Eng 2021; 5: 815–829. doi: 10.1038/s41551-021-00718-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Urbanczyk M, Layland SL, Schenke-Layland K. The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues. Matrix Biol 2020; 85–86: 1–14. doi: 10.1016/j.matbio.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 82.Jaffar J, Yang SH, Kim SY, et al. . Greater cellular stiffness in fibroblasts from patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2018; 315: L59–L65. doi: 10.1152/ajplung.00030.2018 [DOI] [PubMed] [Google Scholar]
- 83.de Hilster RHJ, Sharma PK, Jonker MR, et al. . Human lung extracellular matrix hydrogels resemble the stiffness and viscoelasticity of native lung tissue. Am J Physiol Lung Cell Mol Physiol 2020; 318: L698–L704. doi: 10.1152/ajplung.00451.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knudsen L, Ochs M. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem Cell Biol 2018; 150: 661–676. doi: 10.1007/s00418-018-1747-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Moorsel CHM, van Der Vis JJ, Grutters JC. Genetic disorders of the surfactant system: focus on adult disease. Eur Respir Rev 2021; 30: 200085. doi: 10.1183/16000617.0085-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suki B, Stamenović D, Hubmayr R. Lung parenchymal mechanics. Comp Physiol 2011; 1: 1317–1351. doi: 10.1002/cphy.c100033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takahashi A, Majumdar A, Parameswaran H, et al. . Proteoglycans maintain lung stability in an elastase-treated mouse model of emphysema. Am J Respir Cell Mol Biol 2014; 51: 26–33. doi: 10.1165/rcmb.2013-0179OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suki B, Sato S, Parameswaran H, et al. . Emphysema and mechanical stress-induced lung remodeling. Physiology 2013; 28: 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tschumperlin DJ. Matrix, mesenchyme, and mechanotransduction. Ann Am Thorac Soc 2015; 12: Suppl. 1, S24–S29. doi: 10.1513/AnnalsATS.201407-320MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jamieson RR, Stasiak SE, Polio SR, et al. . Stiffening of the extracellular matrix is a sufficient condition for airway hyperreactivity. J Appl Physiol 2021; 130: 1635–1645. doi: 10.1152/japplphysiol.00554.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maarsingh H, Bidan CM, Brook BS, et al. . Small airway hyperresponsiveness in COPD: relationship between structure and function in lung slices. Am J Physiol Lung Cell Mol Physiol 2019; 316: L537–L546. doi: 10.1152/ajplung.00325.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu F, Mih JD, Shea BS, et al. . Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 2010; 190: 693–706. doi: 10.1083/jcb.201004082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jones MG, Andriotis OG, Roberts JJ, et al. . Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis. eLife 2018; 7: e36354. doi: 10.7554/eLife.36354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raghu G, Brown KK, Collard HR, et al. . Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial. Lancet Respir Med 2017; 5: 22–32. doi: 10.1016/S2213-2600(16)30421-0 [DOI] [PubMed] [Google Scholar]
- 95.Schilter H, Findlay AD, Perryman L, et al. . The lysyl oxidase like 2/3 enzymatic inhibitor, PXS-5153A, reduces crosslinks and ameliorates fibrosis. J Cell Mol Med 2019; 23: 1759–1770. doi: 10.1111/jcmm.14074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bich L, Pradeu T, Moreau JF. Understanding multicellularity: the functional organization of the intercellular space. Front Physiol 2019; 10: 1170. doi: 10.3389/fphys.2019.01170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berankova K, Uhlik J, Honkova L, et al. . Structural changes in the bronchial mucosa of young children at risk of developing asthma. Pediatr Allergy Immunol 2014; 25: 136–142. doi: 10.1111/pai.12119 [DOI] [PubMed] [Google Scholar]
- 98.Bonato M, Tiné M, Bazzan E, et al. . Early airway pathological changes in children: new insights into the natural history of wheezing. J Clin Med 2019; 8: 1180. doi: 10.3390/jcm8081180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hata A, Schiebler ML, Lynch DA, et al. . Interstitial lung abnormalities: state of the art. Radiology 2021; 301: 204367. doi: 10.1148/radiol.2021204367 [DOI] [PMC free article] [PubMed] [Google Scholar]