Fig. 6 |. Cellular architecture of the post-infectious lyme arthritis synovial lesion.
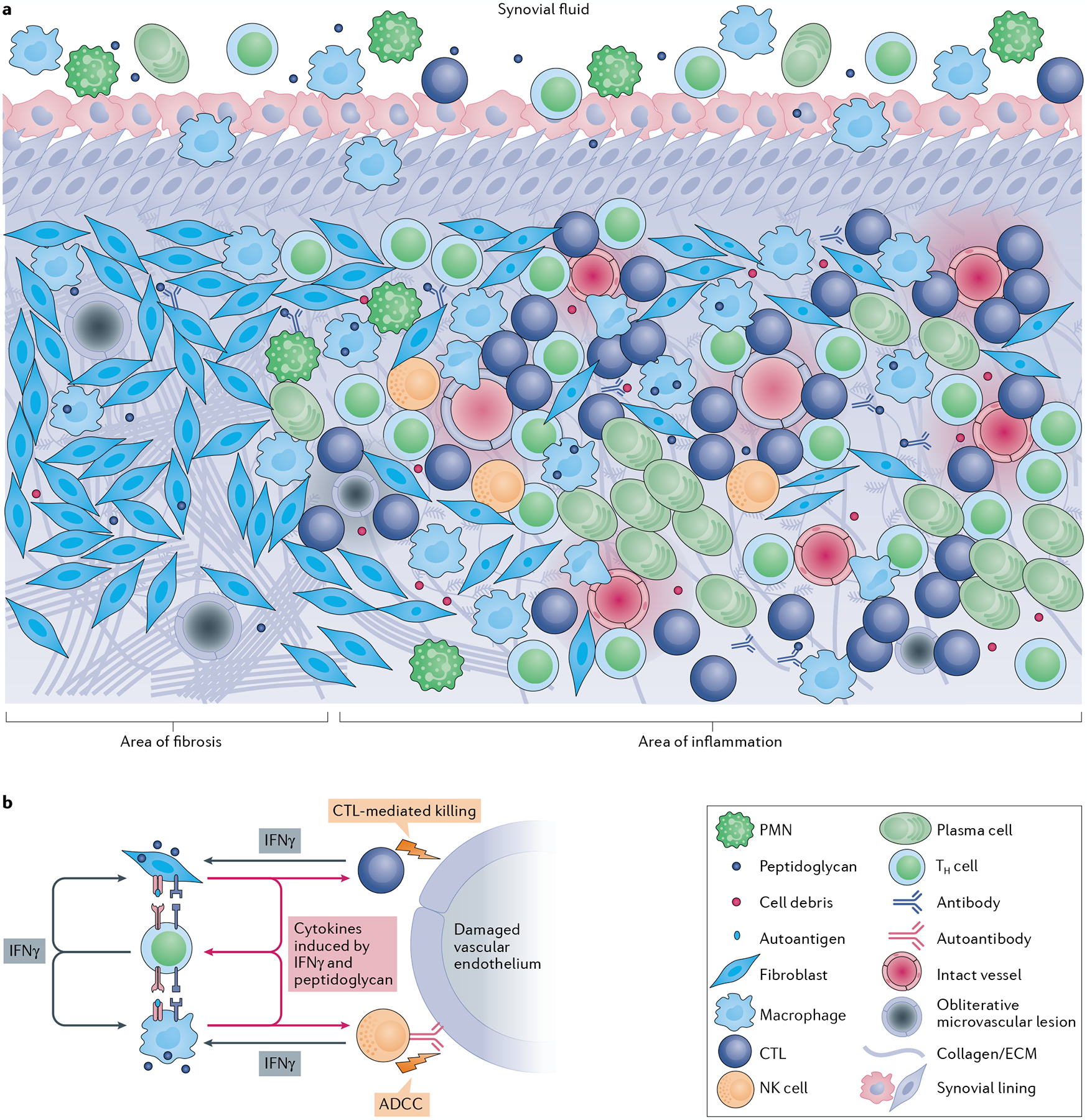
a | The post-infectious Lyme arthritis synovial lesion is characterized by widespread fibrosis and areas of marked inflammation. Fibrotic areas contain large numbers of synovial fibroblasts, obliterative microvascular lesions, disordered collagen and other extracellular matrix (ECM) proteins. Areas of inflammation are found primarily in highly vascularized synovial intimal and subintimal layers that can contain obliterative microvascular lesions and/or intact vessels. Immune cells, such as macrophages, CD4+ T helper (TH) cells, cytotoxic T lymphocytes (CTLs; mostly CD8+ T cells with a few γδ T cells), natural killer (NK) cells, and large numbers of antibody-producing plasma cells, are found primarily in vascularized areas, but can be found throughout the tissue. Vascularized areas also contain many HLA-DR-expressing synovial fibroblasts; however, they tend to have less fibrotic tissue. Bacterial peptidoglycan is present in synovial fluid and might additionally be present in inflamed tissue, along with degraded cellular and ECM debris. Only a few polymorphonuclear cells (PMNs) are present in post-infectious Lyme arthritis synovial tissue, but more are present in synovial fluid. b | In this panel, a hypothesis is developed regarding the roles of important cells and immune mediators in autoimmune-mediated damage to the endothelium in inflamed synovia. Large amounts of IFNγ produced by TH cells, CTLs and NK cells induce potent responses by HLA-DR-expressing synovial fibroblasts and macrophages, which upregulate proteins associated with antigen presentation, T cell activation and inflammation. Borrelia burgdorferi peptidoglycan and cell debris might amplify these responses. Synovial fibroblasts and macrophages present MHC class II-restricted peptides derived from Lyme autoantigens, which are abundant in synovial tissue, to autoreactive TH cells, perpetuating IFNγ responses in the tissue. Endothelial cells, which were damaged during infection with B. burgdorferi, can be targeted for killing by CTLs, either through direct CTL-mediated killing or through autoantibody-dependent cell-mediated cytotoxicity (ADCC), or both. Further damage to the microvasculature releases more autoantigens and debris, leading to a feedback loop of chronic inflammation and tissue damage.
