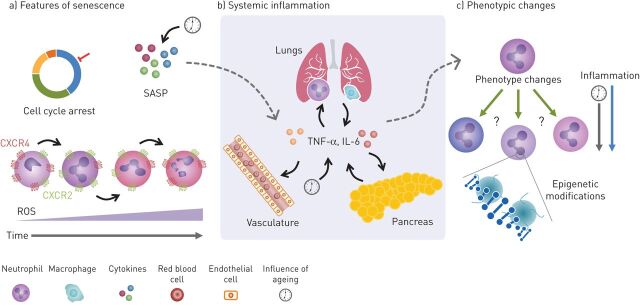
FIGURE 1.
Potential common inflammatory mechanisms between COPD, type-2 diabetes mellitus (T2D) and cardiovascular disease (CVD). a) Senescence is described as changes that result in cell-cycle arrest and is associated with a senescence-associated secretory phenotype (SASP). The SASP leads to increases in inflammatory cytokines both locally and systemically (dotted arrow). This process in neutrophils, while slightly different to typical senescence, might be characterised by increases in chemokine receptor CXCR4 and decreases in CXCR2. This may change the way neutrophils behave and contribute to the damage seen in these diseases. These changes are also associated with increased reactive oxygen species (ROS) production, causing an oxidant imbalance and increasing damage. b) Inflammatory cytokines released by lung epithelial cells and immune cells can enter circulation from the lung in COPD. Likewise, these cytokines can also be released by immune cells in the pancreas in T2D and from endothelium and plaques in CVD. Increases of these cytokines also occur with age and can influence the cytokine milieu, leading to potential changes in neutrophil phenotype (dotted arrow). c) Changes in neutrophil subpopulations (or cell phenotype) caused by inflammation may also play a role in the crosstalk between COPD, CVD and T2D by contributing to tissue damage. There is also some evidence to suggest epigenetic changes may perpetuate the disease state causing long-term maladaptive changes to cellular phenotype and function, influenced by both inflammation and ageing. TNF: tumour necrosis factor; IL: interleukin.