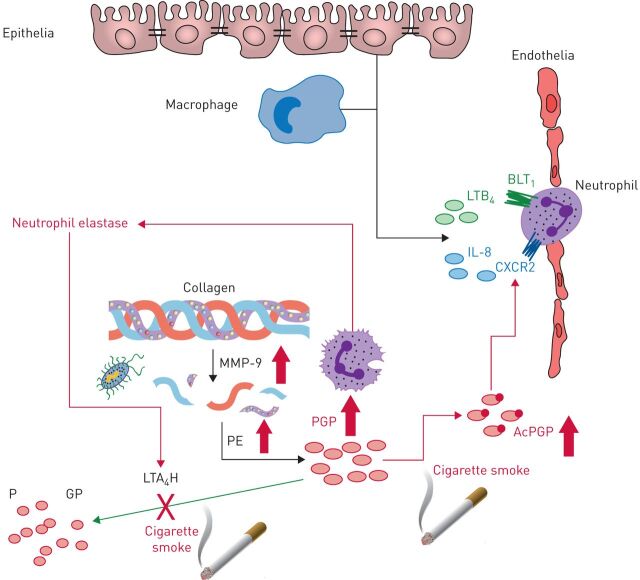
FIGURE 4.
Leukotriene A4 hydrolase (LTA4H)-mediated degradation of Pro-Gly-Pro (PGP) is disturbed in chronic lung diseases. Neutrophils recruited to the lung release enzymes (matrix metalloproteinases (MMPs) and prolylendopeptidase (PE)) that proteolytically target collagen to generate PGP. To counteract this, LTA4H is released to degrade PGP and resolve neutrophilic inflammation. If this balance between PGP-generating and PGP-degrading enzymes is disturbed then PGP can persist and drive pathology. Cigarette smoke, a major risk factor for the development of chronic obstructive pulmonary disease, can promote the expression and/or release of MMPs and PE and ensuing PGP generation. Furthermore, cigarette smoke disturbs the LTA4H-mediated anti-inflammatory pathway in two ways: 1) chemically acetylating PGP (AcPGP) and protecting it from degradation by LTA4H; and 2) selective abrogation of LTA4H PGP-degrading activity. A protease imbalance is a hallmark of many chronic lung diseases, with neutrophil elastase prominently implicated in disease pathologies. Neutrophil elastase can promote MMP activation to facilitate PGP generation, whilst degrading extracellular LTA4H. Respiratory infections act to further spike PGP generation in a setting where degradation is impaired. As a result, AcPGP/PGP can drive neutrophilic inflammation and pathology. LTB4: leukotriene B4; IL: interleukin; BLT1: Leukotriene B4 receptor 1.