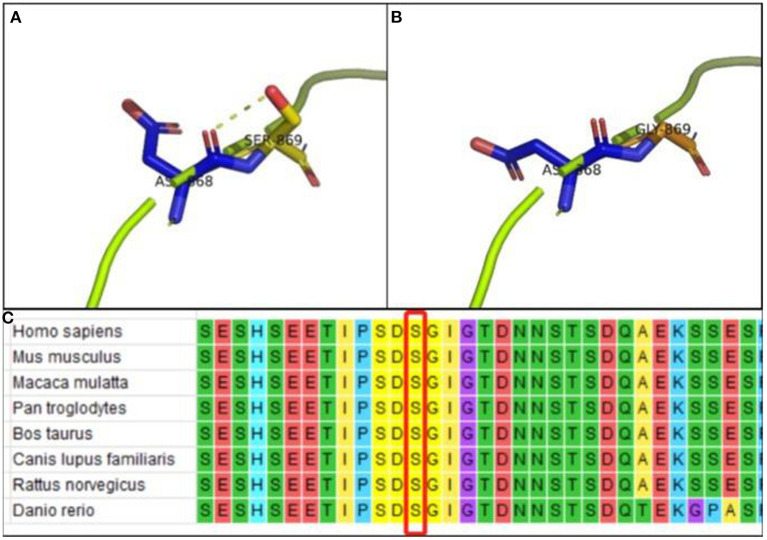
Figure 4.
(A) PyMOL software analysis: Ser869 interacts with Asp868 via hydrogen bonding in wild-type p.Ser869 in SETBP1; (B) PyMOL software analysis: no hydrogen-bond interaction between Gly869 and Asp868 in mutant-type p.Ser869 in SETBP1, indicating that variants affect protein structure and function; (C) Alignment of the SETBP1 sequences revealed that the amino acid residues at position 869 were strictly conserved.