Abstract
Most chronic and acute lung diseases have no cure, leaving lung transplantation as the only option. Recent work has improved our understanding of the endogenous regenerative capacity of the lung and has helped identification of different progenitor cell populations, as well as exploration into inducing endogenous regeneration through pharmaceutical or biological therapies. Additionally, alternative approaches that aim at replacing lung progenitor cells and their progeny through cell therapy, or whole lung tissue through bioengineering approaches, have gained increasing attention. Although impressive progress has been made, efforts at regenerating functional lung tissue are still ineffective. Chronic and acute lung diseases are most prevalent in the elderly and alterations in progenitor cells with ageing, along with an increased inflammatory milieu, present major roadblocks for regeneration. Multiple cellular mechanisms, such as cellular senescence and mitochondrial dysfunction, are aberrantly regulated in the aged and diseased lung, which impairs regeneration. Existing as well as new human in vitro models are being developed, improved and adapted in order to study potential mechanisms of lung regeneration in different contexts. This review summarises recent advances in understanding endogenous as well as exogenous regeneration and the development of in vitro models for studying regenerative mechanisms.
Short abstract
Once thought to be a quiescent organ, we now know the lung demonstrates an incredible capacity for regeneration in response to injuries. However, there is also a growing appreciation for impairments in these processes such as ageing and the diseased niche. https://bit.ly/3hJgAAS
Introduction
The lung is continuously exposed to different environmental insults such as dust, aerosols, smoke and pathogens, which can cause cellular damage. Despite its normally quiescent nature, the lung possesses a potent reparative capacity, in which the resident progenitor cell populations proliferate and differentiate into diverse cell types after injury [1, 2]. Recent findings support the existence of several stem or progenitor cells that are spatially and temporally restricted in the lung, rather than a single progenitor cell able to give rise to all the different pulmonary cells [1, 2]. However, the enormous cellular diversity in the adult lung and the low cell turnover rate have hindered the identification of human lung progenitor cells and most of the available data come from mouse models of lung injury [1, 2]. Despite the similarities in lung development among mammals, there are significant differences in structure, cellular composition, molecular mechanisms and responses between mouse and human lungs [3, 4]. This highlights the need for new human models to study lung regeneration and disease. Here, we will describe the main progenitor populations, their niches and how alterations in these populations in ageing and disease compromise their capacity to regenerate the lung. We will further describe models that might be helpful to understand these processes in the human lung.
Endogenous regeneration
The human respiratory system consists of several epithelial, mesenchymal and immune cells with a defined distribution along the respiratory tract [5]. In the proximal airways of mice and humans, basal epithelial cells act as progenitor cells, giving rise to either secretory or ciliated cells [1, 6, 7]. Studies in mice and humans have shown that the Notch signalling pathway is crucial for basal cell differentiation under physiological conditions or after injury [8–10]. Other progenitor populations, such as club cells and variant club cells, support the homeostasis and repair responses in the rodent lung [1, 5, 6].
More distally, the human epithelium is predominantly composed of club cells and fewer basal and goblet cells [5]. Moreover, single neuroendocrine cells (NECs) are found along the airway and clustered in neuroepithelial bodies (NEBs) at the airway branch points [11]. NECs proliferate, self-renew and replenish club and ciliated cells after injury. Although NECs are not essential for epithelial repair, the NEB microenvironment supports the maintenance of progenitor cell populations in the lung [11, 12]. Conversely, rodent bronchioles are lined by a simple epithelium composed mainly of ciliated and club cells [3]. Furthermore, bronchioalveolar stem cells contribute to the replenishment of both bronchial epithelial and alveolar cells after injury in mice [5, 13, 14]. However, the existence of these cells in human lungs, as well as of other potential progenitor cells, remains to be elucidated.
The alveoli are covered by a thin, single layer of squamous alveolar epithelial type 1 (AT1) cells that are the main site for gas exchange [1, 2, 6] and cuboidal alveolar epithelial type 2 (AT2) cells that have a critical role in the synthesis of surfactant lipids and serve as progenitor cells [1, 2, 5, 6, 15]. In early developmental stages in mice, AT1 and AT2 cells are derived from a bipotent alveolar progenitor [16, 17], while a subset of AT2 cells with active Wnt signalling (Axin2+) serve as progenitor cells during homeostasis and upon injury in adult mice [18]. In line with this, single-cell sequencing revealed a keratin (KRT)8+ intermediate progenitor population derived from AT2 and club cells after injury in mice; KRT8 populations in human lungs showed an enrichment of Wnt signalling [19]. In the alveolar regions, niche fibroblasts maintain the stemness and determine the fate of AT2 cells via paracrine Wnt signalling [18]. This demonstrates the importance of epithelial–mesenchymal crosstalk in regeneration of the lung. Moreover, immune cells are believed to influence progenitor cell function; however, our understanding of immune–progenitor cell crosstalk in the lung is in its infancy.
The response after injury varies along the pulmonary tract and depends not only on the persistent exogenous insults but also on endogenous factors such as genetic alterations or ageing. Given the fact that most chronic lung diseases are related to ageing [6, 20], a better understanding of the changes in regeneration processes in the ageing lung is critical for the development of new therapeutic options.
Regeneration of the ageing lung
Ageing is a natural process composed of physiological and molecular changes leading to progressive impairment of organ function [2, 6, 20]. In the lung, ageing diminishes pulmonary remodelling and regeneration capacity. Age-associated hallmarks include telomere shortening, mitochondrial dysfunction, cellular senescence and stem cell exhaustion [2, 20]. Most hallmarks are interconnected and drive each other, thereby contributing to the inability of the lung to respond to injuries [2].
Accordingly, aged mice are more susceptible to lung injury caused by cigarette smoke or bleomycin [21–23]. For example, aged bleomycin-treated mice showed increased transforming growth factor (TGF)-β1 secretion, excessive extracellular matrix (ECM) deposition and increased recruitment of circulating fibrocytes [2, 21, 24]. Notably, bone marrow-derived mesenchymal stromal cells (B-MSCs) derived from young mice have a protective role against fibrosis, whereas aged B-MSCs had a lower regenerative capacity [21, 24, 25].
Cellular senescence is characterised by an irreversible cell cycle arrest along with a pro-inflammatory senescence-associated secretory phenotype (SASP) [26]. Under physiological conditions, accumulating senescent cells are readily cleared to promote tissue regeneration [27, 28]. However, upon chronic damage or ageing, senescent cells persist in the tissue, contributing to impaired regeneration [2, 26, 29, 30]. Evasion of clearance mechanisms is potentially mediated by a change in SASP [31] or by age-related immune system dysfunction (immunoageing) [32]. Accordingly, increased autoimmunity, reduced pathogen clearance, and chronic low-grade sterile inflammation (inflammageing) are characteristics of the ageing lung [33–35]. Increased DNA damage and mitochondrial dysfunction induce senescence in B-MSCs, which show a reduced protective ability in idiopathic pulmonary fibrosis (IPF) patients [36] and induce senescence and collagen deposition in fibroblasts via their SASP [2]. Similarly, fibroblasts derived from IPF patients show increased cellular senescence markers and differentiation rate towards myofibroblasts and a lower response to TGF-β [37–39]. In line with these findings, bleomycin-treated mice display an accumulation of senescent myofibroblasts [23], with an increased expression of the nicotinamide adenine dinucleotide phosphate oxidase-4 (Nox4) and impaired NFE2-related factor 2 (Nrf2) antioxidative responses in the lung. Thus, elevated levels of Nox4 and reduced levels of Nrf2 in IPF lungs might contribute to nonresolving fibrosis.
In the alveolar region, the main progenitor cells (AT2 cells) become senescent in older mice, losing their progenitor capacity [40]. Under physiological conditions, the Wnt signalling pathway is required for AT2 progenitor cell function and tissue homeostasis [40, 41], while aberrantly activated Wnt/β-catenin signalling in AT2 cells has been related to IPF pathogenesis and cellular senescence [40, 42]. Moreover, AT2 cells in older mice exhibit higher Nrf2-mediated oxidative stress responses and mammalian target of rapamycin (mTOR) activity [43]. This suggests that aberrant activation of developmental pathways might drive premature ageing phenotypes, thereby compromising the regenerative potential of the lung. Age-related changes are also observed in the bronchiolar epithelium, leading to a compromised clearance capacity in the proximal airways characterised by increased apoptosis, abnormal cilia and altered mucus [2, 43]. Moreover, as shown for AT2 cells, a redox imbalance indicated by lower Nrf2-mediated oxidative stress response and higher mTOR signalling has been described for club cells, a main progenitor cell in proximal airways [43]. In conclusion, inflammageing, cellular senescence and stem cell depletion contribute to impaired tissue regeneration in the aged lung. Further studies regarding the contribution of rare progenitor populations in the aged human lung are needed.
Regeneration of a diseased lung
Chronic diseases
Idiopathic pulmonary fibrosis
IPF, a chronic, progressive, irreversible and usually lethal disease, is the most common interstitial lung disease [44]. Most IPF patients are elderly (median age ∼66 years) with a poor survival rate and lung transplantation is the only curative treatment [44]. Although IPF pathogenesis is not fully understood, it has been proposed that an abnormal wound healing process after repetitive insults to the epithelium might compromise the regenerative potential of the lung, thereby promoting fibrotic changes [20, 45].
Age-related cellular changes in IPF patients, such as telomere shortening and increased oxidative stress and senescence, suggest a strong connection between premature ageing mechanisms and pulmonary fibrosis [30, 44]. AT2 cells show reduced progenitor potential in aged lungs challenged with influenza [46] and have been described as the main target of apoptosis and premature senescence in IPF [30, 45, 47]. Additionally, the accumulation of abnormal surfactant proteins inside AT2 cells (or persistent viral infections) activates the unfolded protein response, thereby promoting apoptosis of AT2 cells and subsequent fibrogenesis in mice [48]. Moreover, the main regulator of the endoplasmic reticulum (ER) response in AT2 cells, the chaperone GRP78, is downregulated with ageing, rendering the alveolar epithelium more prone to ER-dependent apoptosis [49]. Additionally, re-epithelialisation of the lung is compromised by reprogramming of AT2 cells along with the acquisition of mesenchymal markers partially induced by ER-dependent TGF-β activation [50]. Furthermore, senescent AT2 cells induce a fibroblast-to-myofibroblast transition (FMT) in surrounding fibroblasts via TGF-β1 [51], Nanog [52], mTOR [53] and Wnt [40, 42] pathways, processes that have been related to exaggerated ECM deposition in IPF lungs [54, 55].
Bronchial epithelial cells can re-epithelialise the alveolar regions after exaggerated loss of alveolar epithelium [56]. Surprisingly, increased senescence markers and Sirtuin 6 expression have been described in the aberrantly activated bronchial epithelial cells lining the cystic lesions of IPF patients [56]. These cells are susceptible to TGF-β-induced senescence and their high interleukin (IL)-1β secretion was sufficient to induce FMT in vitro [56]. Moreover, several recent studies have utilised single-cell RNA sequencing to explore the diversity of cell types and aberrant transcriptional programmes in IPF. Despite the fact that cells were sequenced from small biopsies of individual patients, these studies all identified the emergence of novel epithelial populations in the lungs of patients with IPF [57–59]. One such novel population recently identified in two independent studies is a KRT5−/KRT17+ population that co-expresses other basal cell, mesenchymal and senescence markers [58, 59]. This cell population also expressed elevated levels of profibrotic and ECM genes and is localised on the epithelium overlying fibrotic foci in IPF lungs (termed aberrant basaloid cells in one study) [58]. While aberrant basaloid cells were not initially classified as an independent cell type in the first single-cell RNA sequencing cohort [57], these cells were observed in a subsequent work by reanalysing the data [58], further reinforcing their existence with relative abundance in IPF patients. This cell population presumably acts as a secondary progenitor after AT1 and AT2 depletion, as observed for rodent bronchioalveolar stem cells.
In conclusion, senescence and SASP signals seem to modulate the epithelial–mesenchymal crosstalk in an unsuccessful attempt at repair in the lung, highlighting their important role in the pathobiology of IPF. Consistently, depletion of senescent cells using senolytic drugs or genetic models has a beneficial effect against fibrosis and other age-related diseases in mice [29, 30, 51, 60]. However, some (pre-)senescent cells seem to be essential for homeostasis and repair in the mouse [61, 62], highlighting the importance of understanding how cell-type-specific senescence modulates IPF.
Chronic obstructive pulmonary disease
COPD is a heterogeneous disease affecting both the upper and lower airways and has a poor prognosis [63, 64]. Similarly to IPF, lung transplantation is the only therapeutic option for end-stage patients. The main pathogenic features are airway inflammation and remodelling, increased mucus production, peri-bronchial fibrosis and loss of alveolar architecture, leading to impaired lung function [64]. The impaired regenerative capacity of the lung observed in COPD patients is linked to a mixture of genetic and external factors, such as indoor and outdoor pollution, cigarette smoking, respiratory infections and occupational exposures [64]. Moreover, given the increasing incidence of COPD with age, and the acceleration of ageing-related processes by smoking, ageing plays a role in COPD pathogenesis [65]. Accordingly, emphysematous changes have been described in multiple accelerated ageing models [65–71], which also show a higher susceptibility to development of emphysema after acute cigarette smoke exposure [68, 70]. However, it is important to highlight that these accelerated ageing models do not fully recapitulate the accumulation of injuries seen during the natural ageing process [64, 72].
COPD patients have significantly shorter telomeres in the lung and in the peripheral circulation compared to age-matched healthy donors [47, 73]. Moreover, autophagy and proteostasis are impaired in COPD patients, thereby promoting cellular senescence in alveolar epithelial cells [64, 65]. Increases in senescence markers have also been observed in mesenchymal populations such as endothelial cells and fibroblasts, suggesting that the altered parenchymal remodelling observed in COPD might be related to a senescence-associated mesenchymal dysfunction [74, 75]. Moreover, primary small airway epithelial cells derived from COPD patients show increased oxidative stress and telomere damage-induced senescence, along with highly activated pro-inflammatory NF-κB signalling upon cigarette smoke exposure [64, 76–78]. Furthermore, impaired immune responses characterised by reduced clearance capacity and higher susceptibility to infections are related to the accumulation of senescent T-lymphocytes and macrophages in COPD lungs [64]. Although studies depleting senescent cells using genetic models have generated conflicting results [79–81], the pharmacological depletion of senescent cells using the senolytic drug ABT-263 conferred resistance to elastase-induced emphysema in both young and aged mice [79].
Contrary to IPF, signalling pathways such as canonical Wnt/β-catenin and Notch are downregulated in the epithelium in COPD [82]. Alterations in these pathways might reduce proliferation and differentiation potential of epithelial progenitors in the lung [63, 82, 83]. Notably, an increase in non-canonical WNT5A/5B signalling in fibroblasts has been observed in aged and COPD lungs, and inhibition of WNT5A/5B reduces lung tissue destruction and improves progenitor potential of AT2 cells in mouse models [63]. The role of TGF-β in COPD seems to be cell type specific, with TGF-β expression being activated in the airway epithelium of smokers, which might explain the fibrotic airway remodelling in these areas and the loss of alveolar structures in the distal lung [84, 85]. Conversely, alveolar macrophages release lower levels of TGF-β1 in COPD, suggesting an impaired anti-inflammatory response in the lung [86]. Moreover, proliferation and elastin production in response to TGF-β1 is impaired in COPD fibroblasts, indicating a lower repair capacity in COPD [85, 87].
Acute disease
Acute respiratory distress syndrome (ARDS) is the main acute condition to be considered in the context of lung regeneration. ARDS is a major health concern worldwide [88], exemplified by the current coronavirus disease 2019 (COVID-19) pandemic. The incidence of ARDS has been estimated at 10–86 cases per 100 000; however, it is speculated that the condition is underrecognised [89]. ARDS is a critical condition associated with a high fatality rate [90], with only moderate improvements during the last decades and increased mortality in elderly patients [91]. The initial lung injury leading to ARDS may be caused by numerous factors, such as infection, gastric aspiration, hyperoxia, mechanical stress (e.g. ventilator-induced) or adverse drug/chemical reactions; however, infection is by far the most common underlying factor for development of ARDS [92]. In addition, some patients undergoing lung transplantation experience acute lung injury within the first 72 h (primary graft dysfunction). While a distinct clinical entity, primary graft dysfunction shares many clinical features and is radiographically similar to ARDS [93]. Histologically, patients with ARDS typically display diffuse alveolar damage (DAD) [94]. On a tissue level, ARDS is characterised by an exudative phase with rapid recruitment of leukocytes, acute inflammation, pneumocyte/endothelial cell death, hyaline membrane formation and oedema, followed by an organising/proliferative phase with AT2 hyperplasia and loose organising fibrosis [89].
Around 70% of the COVID-19-related deaths have been due to severe ARDS [95]. Some reports have additionally reported DAD or DAD-like histological patterns in COVID-19 patients [96–99]. While most patients surviving the acute phase of ARDS transition to a resolving phase, returning the lung parenchyma to a (near) normal state, some patients transition into a fibrosing phase [100, 101]. The fact that the lung tissue is restored in most patients after the massive ARDS-associated damage highlights the regenerative potential of the lung. There are several mechanisms that are critical in ARDS-associated lung regeneration, including oedema re-absorption (i.e. re-establishment of effective ion transport by the alveolar epithelium and re-formation of tight junctions), resolution of inflammation (e.g. regulation of crosstalk between the innate and adaptive immune systems), cell proliferation/differentiation to re-populate the damaged epithelium and/or endothelium (e.g. proliferation and differentiation of AT2 cells), and remodelling of the ECM [102–104]. However, the endogenous repair of the lung on a molecular level after ARDS and why some patients develop chronic fibrosis is still incompletely understood.
Exogenous regeneration
Bioengineering
The only available treatment for end-stage lung disease remains lung transplantation. There are not enough donor lungs to meet current or anticipated future needs. Approximately 5000 lung transplants are performed annually worldwide, including 1000 in Europe, with equal numbers of patients on waiting lists [105]. Transplantation recipients require lifelong immunosuppression and 5-year survival after lung transplantation remains only ∼50%. New options to increase the number of donor lungs are desperately needed.
A promising area of research is the (re)generation of pulmonary tissue using ex vivo bioengineering methods. In these approaches, cells are combined with a scaffold outside of the body, with the goal of forming new tissue for transplantation [106]. Scaffolds can be derived from either biological or artificial materials (or a combination thereof in hybrid approaches) and could be seeded with an appropriate cell source to regenerate functional lung tissue for subsequent transplantation. While these techniques are still not fully developed, one of their purported benefits is that scaffolds could be re-cellularised with autologous cells derived from the eventual transplant recipient, thus minimising the immunological complications typically accompanying lung transplantation. Similar approaches have already been used clinically for structurally simpler tissues such as skin and cardiac valves [107].
Early work has focused on proof-of-concept studies in rodents, including transplantation of bioengineered grafts. Major advances have also been made with regard to deriving biological scaffolds from human and porcine lungs, while research using synthetic or engineered materials remains at an earlier stage [106]. Protocols to consistently de-cellularise lungs from a variety of species (including rodent, porcine and human) have been developed, while the development of synthetic and engineered scaffolds is still relatively in its infancy [106]. Acellular lung scaffolds support the viability of diverse primary stem and progenitor cells over several weeks. Importantly, bioreactors that support human- and porcine-sized scaffolds have been developed and used for studying the potential for re-endothelialisation and re-epithelialisation [108, 109]. One of the major challenges of these studies is growing a sufficient number of cells to cover the endothelial or epithelial surfaces of acellular human or porcine lungs. Several different approaches have been described recently for growing large amounts of induced pluripotent stem cells for deriving endothelium [109] as well as primary basal epithelial progenitor cells from the proximal [110] and distal lung [108], which retain the potential for differentiation into multiple cell types found in the lung epithelium. Furthermore, porcine scaffolds re-cellularised with human cells have been transplanted into porcine recipients (left lung only) [111, 112] and animals survived for up to 1 month [111], demonstrating the potential of this approach. However, transplanted grafts were not fully functional and there was evidence of aberrant remodelling over time [111]. While these are important advances, we are still far away from generating tissue for clinical translation. As the lung is composed of over 40 cell types, further advances are needed to generate tissue that contains the diversity of cell types seen in normal lung tissue and that can function over time.
Cell therapy
Cell therapy approaches seek either to replace damaged tissue through delivery and engraftment of administered cells or to stimulate endogenous repair and regeneration via immunomodulation. A variety of cell types and cell-derived products are currently being explored, with many showing a remarkable capacity to contribute to lung repair in pre-clinical small and large animal models [113–117]. Owing to their success in pre-clinical models, several different cell types have been explored in clinical trials for lung diseases. B-MSCs have been the most widely explored in clinical trials for ARDS and COPD and their potential for immunomodulation, but there have been no reports of clear efficacy in human trials [118]. One area of intense discussion is the suitability of the pre-clinical animal models and the timing of MSC administration [119]. To date, most animal studies have been performed in young animals, which are known to have a high capacity for regeneration. Clinically, the overwhelming majority of patients with acute and chronic lung disease are older and the baseline capacity for repair is known to be different. In addition, there are open questions with regard to how best to scale cell-dosing strategies for human trials and the effect of manufacturing, including cell freezing and thawing [120].
In addition to MSCs, endogenous progenitor cells and induced pluripotent derived endogenous progenitor cells (e.g. epithelial, endothelial and alveolar macrophage) are being explored for replacing damaged resident lung cells [118, 119, 121]. These cell types have been explored to a more limited extent in pre-clinical models than MSCs, but some have shown promise in different acute and chronic lung disease animal models. AT2 cells have been explored in a small cohort of patients with IPF [122], and basal epithelial progenitor cells in bronchiectasis [123]. While no adverse side-effects were reported in either of these clinical studies, cells were administered to a small number of patients and the studies therefore provide limited insight into the potential for efficacy. More mechanistic studies in relevant pre-clinical models showing efficacy, including in humanised models, are critical for moving cell therapy safely into the clinic.
Models of lung disease and regeneration
A variety of in vitro models have emerged in recent years to better understand the biology associated with lung regeneration as well as to evaluate potential therapies. Major biological and physiological differences exist in the lungs between species. Thus, development of humanised models is critical. While isolated primary human cells can be used to study lung disease and regeneration in vitro, these studies do not mimic the complexity and communication between cell types found in vivo. Several different three-dimensional models have emerged in the last few years and each have different strengths and weaknesses that are important to consider when using them to study lung regeneration. Here we will focus on organoids, acellular lung scaffolds and precision-cut lung slices (PCLS), as these have been most extensively used to study lung disease and regeneration. However, other models such as air–liquid interface cultures, lung-on-a-chip and engineered systems on the macro scale, which use human tissue or cells, have also shown promise and have been reviewed elsewhere [124, 125].
Organoids
Organoids have been widely used to characterise stem cell function and regenerative potential of different progenitor cells in human and rodent lungs. Typically, primary cells are isolated by cell sorting using specific cell markers and seeded in the presence or absence of mesenchymal cells on an anti-adherent substrate such as Matrigel [126]. Recently, human induced pluripotent stem cell-derived organoids were developed, allowing the modelling of several diseases including cystic fibrosis [127], cancer or pulmonary fibrosis [128]. Depending on the application, different epithelial progenitor cells can be used for organoid formation. Rodent basal cells can self-renew and give rise to differentiated ciliated cells by cultivation without stromal cells to obtain bronchospheres [129]. Moreover, human airway organoids can be derived by isolating primary airway basal cells and obtaining human bronchospheres composed of ciliated, goblet and basal cells [110, 130] or a mixture of human adult primary bronchial epithelial cells, lung fibroblasts and lung endothelial cells [131]. These culturing methods show a high efficiency in obtaining and expanding the organoids, allowing high-throughput screening for regenerative applications [110, 130]. Furthermore, the progenitor capacity and their response to injury of mouse cell populations in the proximal airways, such as club cells, was determined using organoid assays [132]. Additionally, organoid platforms have been used to study rare bipotent bronchial alveolar progenitors, highlighting the importance of the epithelial–mesenchymal or epithelial–endothelial crosstalk in progenitor activation and tissue repair after injury [133, 134].
AT2 cells isolated using either genetic lineage tracing [126] or antibody labelling of surface markers for mice [132, 133, 135] or humans generally require the presence of mesenchymal cells to form organoids [132, 133, 135]. Additionally, the fact that AT2 cells cannot currently be expanded without losing their progenitor phenotype limits their application in high-throughput studies [126]. However, murine alveolar organoids have been very useful to study age-related changes [40, 63, 136] and how they impair progenitor cell potential and epithelial–mesenchymal crosstalk [137]. Recently, organoid assays led to the discovery of a murine epithelial progenitor population that requires low Wnt/β-catenin signalling, which is impaired in emphysema [41]. Likewise, transient inhibition of the retinoic acid pathway followed by histone deacetylase inhibition was identified as a potential therapeutic option for regenerative treatments [138]. Alveolar organoids derived from human pluripotent cells have also been used as a model for pulmonary fibrosis, identifying the role of the pro-inflammatory cytokine IL-11 [128]. In general, organoid assays allow the discovery of progenitor populations and the study of regenerative mechanisms. However, the isolated populations are typically heterogeneous, making it challenging to attribute organoid formation capacity to a specific subpopulation, especially under conditions of disease that might change cell composition or cell fates. Moreover, even with advances in organoids, they fail to recapitulate the complexity of the distal lung, as immune cells and endothelial cell interactions mostly remain absent.
Acellular lung scaffolds and cell-derived ECM
The role of the ECM and local stem cell niche in regulating repair and regeneration under homeostatic and diseased conditions has recently received increased attention. Historically, this has been challenging to study due to a lack of experimental techniques to study interactions between cells and the ECM in disease-relevant contexts. Acellular scaffolds have recently emerged as a valuable model and can be derived by de-cellularising normal and diseased lungs across species, including human lungs [139], or alternatively derived from de-cellularisation of monocultures [140]. In contrast to scaffolds that include only one or two ECM components or ECM-mimicking binding sites, acellular scaffolds derived at the tissue or cell level retain diverse ECM components and associated growth factors that represent the complexity of the in vivo environment [46, 141, 142]. Scaffolds can be re-cellularised with one or more cell types derived from normal or diseased lung tissue and studied for up to 1 month. Thus far, a variety of different cell types have been seeded onto acellular scaffolds, including distal and proximal epithelial cells, macrophages, fibroblasts, MSCs derived from different tissues and induced pluripotent stem cell-derived endothelial and epithelial cells [139].
While some studies have included multiple cell types in re-cellularised acellular scaffolds, the majority of studies have only included one or two cell types and it remains challenging to direct re-cellularised populations to the exact location where they are found in the lung (e.g. interstitial fibroblasts). Incorporation of more cell populations in these models, as well as directing them to their native locations to reconstruct the appropriate cell–niche interactions, are important challenges to overcome in order to mimic more closely the native tissue environment. Nonetheless, these studies have outlined the critical role that the environment and niche play in directing repair and regenerative processes in several chronic lung diseases, including IPF, COPD and pulmonary hypertension, as well as ageing [143]. The majority of these studies indicate that changes in the microenvironment are major drivers of disease and may play a role in inhibiting normal regeneration. Normal cells seeded into diseased environments change their phenotype and lose function, mimicking changes seen in diseased patients [140–142, 144]. Conversely, cells isolated from diseased patients and seeded into environments mimicking normal lung lose some disease-like phenotypes [144]. These studies and others point to a previously underrecognised degree of cellular plasticity in patients with chronic lung disease, whereby diseased cells can reacquire a more normal state if placed in the right environment. To date, the majority of drugs explored for repair or regeneration have focused on modulating cell behaviour. Targeting the environment may be a more promising approach and the use of de-cell and re-cell systems could play an important role for drug discovery as they are readily amenable to high-throughput studies [141] and can be performed using human tissue that mimics patient disease, including patient heterogeneity.
Precision-cut lung slices
PCLS derived from several species have recently attracted increased attention as models of disease [145], due to the fact that they conserve the architecture and cellular diversity of the lung and remain viable ex vivo for several days [145]. One major disadvantage of PCLS is the absence of recruited immune cells. However, some immune cells, such as alveolar macrophages, dendritic cells or mast cells, are still present and their interaction with the residing cells could be studied in mouse and human PCLS [145, 146]. This suggests that this model could be adapted to study certain immune–structural cell crosstalk upon viral, bacterial or fungal infections in the future. Notably, post-natal alveologenesis can be monitored and manipulated ex vivo using PCLS derived from mice [147], highlighting the potential of this ex vivo model to study regenerative processes in real time [148]. Moreover, fibrotic changes in the lung can be studied on PCLS derived from mice and rats previously treated with bleomycin, or PCLS generated from IPF patients, which both maintain fibrotic changes in ECM components as well as soluble mediators, as observed in vivo [149, 150]. These PCLS models have helped in the identification and characterisation of potential novel therapeutic compounds, such as a mTOR/phosphoinositide 3-kinase (PI3K) inhibitor, or in identifying previously unknown effects of known drugs such as nintedanib or pirfenidone (e.g. on the alveolar epithelium) [149, 150]. Notably, specific depletion of senescent cells using senolytic drugs reduced SASP factor secretion and ECM markers in fibrotic mouse PCLS [30]. A potential role of canonical Wnt/β-catenin and WNT5A/5B in fibrosis and COPD, respectively, was also investigated using PCLS [40, 83].
The induction of fibrotic-like changes ex vivo has recently been achieved with a “fibrotic cocktail” composed of several cytokines and growth factors present in the IPF disease milieu that was used to treat PCLS derived from tissue without interstitial lung disease, resulting in increased expression of fibrotic markers of the mesenchyme, losses in phenotypic markers of epithelial progenitor cells, and deposition of ECM proteins (e.g. collagen) [151]. Similar to IPF, PCLS isolated from COPD patients maintain their phenotype in culture and pharmacological activation of Wnt/β-catenin signalling induces repair [152]. Altogether, PCLS are emerging as a tool to bridge the gap between target identification and translation, allowing for future precision medicine approaches.
Challenges and future directions
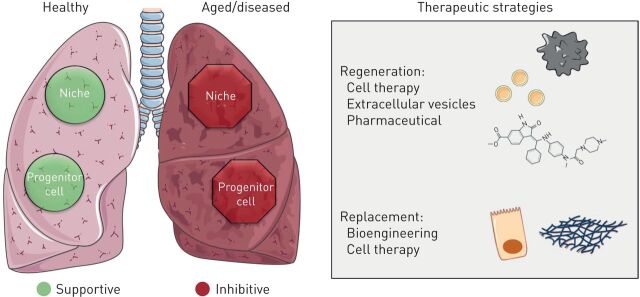
Promising approaches, both in the field of bioengineering and exogenous lung regeneration as well as in the activation of endogenous regenerative potential, have been put forward in recent years. The ongoing COVID-19 pandemic highlights the need for a better understanding of lung regeneration in the face of overwhelming acute viral injury. Specific challenges in these approaches are not only the impaired progenitor cell function but the destructive niche and ECM in aged and diseased lung (figure 1). Reprogramming of the niche by removing prematurely aged cells or reverting the immunoageing phenotype in the aged or diseased lung, employing either cell therapy or pharmacological compounds, will be essential for allowing efficient regeneration and might be efficient in combination with other cell therapy and bioengineering approaches. In order to achieve this goal, (human) models recapitulating the complexity of cell–cell interactions, ECM and the instructive niche are promising tools for furthering our understanding of activating lung regeneration and developing strategies to therapeutically activate and support these processes to target chronic and acute lung diseases.
FIGURE 1.
Therapeutic strategies to repair the diseased lung. Functional progenitor cells and instructive niche promote repair and regeneration in the healthy lung (green). Conversely, progenitor cell dysfunction and inhibitory factors in the niche impair proper lung regeneration and promote disease in the aged/diseased lung (red). Novel therapeutic strategies based on tissue regeneration and/or replacement aim to cure acute and chronic lung diseases by recovering progenitor cell function and/or reprogramming the niche environment. Tissue regeneration could be stimulated with cell therapy, extracellular vesicles or through pharmaceutical approaches targeting the niche or progenitor cells. Alternatively, diseased or damaged cells could be replaced via cell therapy or through bioengineering new tissue or niches. Reproduced and modified from Servier Medical Art with permission.
Footnotes
Provenance: Commissioned article, peer reviewed.
Conflict of interest: M.C. Melo-Narváez has nothing to disclose.
Conflict of interest: J. Stegmayr has nothing to disclose.
Conflict of interest: D.E. Wagner reports grants from the Knut and Alice Wallenberg Foundation and the Swedish Research Council, during the conduct of the study; and personal fees from Boehringer Ingelheim, outside the submitted work. In addition, D.E. Wagner has a patent WO2014169111A1 pending.
Conflict of interest: M. Lehmann reports grants from The German Federal Institute for Risk Assessment (BfR), during the conduct of the study.
Support statement: Funding was received from Bundesinstitut für Risikobewertung (Grant “Lung aging in a dish”), the Swedish Research Council and Knut och Alice Wallenbergs Stiftelse. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med 2014; 20: 822–832. doi: 10.1038/nm.3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho SJ, Stout-Delgado HW. Aging and lung disease. Annu Rev Physiol 2020; 82: 433–459. doi: 10.1146/annurev-physiol-021119-034610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolić MZ, Sun D, Rawlins EL. Human lung development: recent progress and new challenges. Development 2018; 145: dev163485. doi: 10.1242/dev.163485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan H, Deutsch GH, Wert SE. Comprehensive anatomic ontologies for lung development: a comparison of alveolar formation and maturation within mouse and human lung. J Biomed Semantics 2019; 10: 18. doi: 10.1186/s13326-019-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee RE, Miller SM, Randell SH. Adult pulmonary epithelial stem cells and their niches. In: Reis RL, ed. Encyclopedia of Tissue Engineering and Regenerative Medicine. Oxford, Academic Press, 2019; pp. 319–336. [Google Scholar]
- 6.Navarro S, Driscoll B. Regeneration of the aging lung: a mini-review. Gerontology 2017; 63: 270–280. doi: 10.1159/000451081 [DOI] [PubMed] [Google Scholar]
- 7.Morrisey EE. Basal cells in lung development and repair. Dev Cell 2018; 44: 653–654. doi: 10.1016/j.devcel.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Rock JR, Gao X, Xue Y, et al. . Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 2011; 8: 639–648. doi: 10.1016/j.stem.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul MK, Bisht B, Darmawan DO, et al. . Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell Stem Cell 2014; 15: 199–214. doi: 10.1016/j.stem.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardo-Saganta A, Law BM, Tata PR, et al. . Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 2015; 16: 184–197. doi: 10.1016/j.stem.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song H, Yao E, Lin C, et al. . Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci USA 2012; 109: 17531–17536. doi: 10.1073/pnas.1207238109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds SD, Giangreco A, Power JH, et al. . Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 2000; 156: 269–278. doi: 10.1016/S0002-9440(10)64727-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim CFB, Jackson EL, Woolfenden AE, et al. . Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005; 121: 823–835. doi: 10.1016/j.cell.2005.03.032 [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, Liu K, Cui G, et al. . Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet 2019; 51: 728–738. doi: 10.1038/s41588-019-0346-6 [DOI] [PubMed] [Google Scholar]
- 15.Evans KV, Lee JH. Alveolar wars: the rise of in vitro models to understand human lung alveolar maintenance, regeneration, and disease. Stem Cells Transl Med 2020; 9: 867–881. doi: 10.1002/sctm.19-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treutlein B, Brownfield DG, Wu AR, et al. . Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 2014; 509: 371–375. doi: 10.1038/nature13173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank DB, Penkala IJ, Zepp JA, et al. . Early lineage specification defines alveolar epithelial ontogeny in the murine lung. Proc Natl Acad Sci USA 2019; 116: 4362–4371. doi: 10.1073/pnas.1813952116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nabhan A, Brownfield DG, Krasnow MA, et al. . A single cell Wnt signaling niche maintains stemness of alveolar type 2 cells. Science 2018; 359: 1118–1123. doi: 10.1126/science.aam6603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strunz M, Simon L, Ansari M, et al. . Longitudinal single cell transcriptomics reveals Krt8+ alveolar epithelial progenitors in lung regeneration. bioRxiv 2019; preprint [ 10.1101/705244]. [DOI] [Google Scholar]
- 20.Meiners S, Eickelberg O, Königshoff M. Hallmarks of the ageing lung. Eur Respir J 2015; 45: 807–827. doi: 10.1183/09031936.00186914 [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Gonzalez ET, Iyer SS, et al. . Use of senescence-accelerated mouse model in bleomycin-induced lung injury suggests that bone marrow-derived cells can alter the outcome of lung injury in aged mice. J Gerontol A Biol Sci Med Sci 2009; 64: 731–739. doi: 10.1093/gerona/glp040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John-Schuster G, Günter S, Hager K, et al. . Inflammaging increases susceptibility to cigarette smoke-induced COPD. Oncotarget 2016; 7: 30068–30083. doi: 10.18632/oncotarget.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hecker L, Logsdon NJ, Kurundkar D, et al. . Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med 2014; 6: 231ra47. doi: 10.1126/scitranslmed.3008182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bustos ML, Huleihel L, Kapetanaki MG, et al. . Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med 2014; 189: 787–798. doi: 10.1164/rccm.201306-1043OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapetanaki MG, Mora AL, Rojas M. Influence of age on wound healing and fibrosis. J Pathol 2013; 229: 310–322. doi: 10.1002/path.4122 [DOI] [PubMed] [Google Scholar]
- 26.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 2014; 15: 482–496. doi: 10.1038/nrm3823 [DOI] [PubMed] [Google Scholar]
- 27.Krizhanovsky V, Yon M, Dickins RA, et al. . Senescence of activated stellate cells limits liver fibrosis. Cell 2008; 134: 657–667. doi: 10.1016/j.cell.2008.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang TW, Yevsa T, Woller N, et al. . Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011; 479: 547–551. doi: 10.1038/nature10599 [DOI] [PubMed] [Google Scholar]
- 29.Schafer MJ, White TA, Iijima K, et al. . Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 2017; 8: 14532. doi: 10.1038/ncomms14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehmann M, Korfei M, Mutze K, et al. . Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J 2017; 50: 1602367. doi: 10.1183/13993003.02367-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira BI, Devine OP, Vukmanovic-Stejic M, et al. . Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat Commun 2019; 10: 2387. doi: 10.1038/s41467-019-10335-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ovadya Y, Landsberger T, Leins H, et al. . Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun 2018; 9: 5435. doi: 10.1038/s41467-018-07825-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 2014; 69: Suppl. 1, S4–S9. doi: 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 34.Metcalf TU, Cubas RA, Ghneim K, et al. . Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 2015; 14: 421–432. doi: 10.1111/acel.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Jiao Y, Fan EK, et al. . Aging-impaired filamentous actin polymerization signaling reduces alveolar macrophage phagocytosis of bacteria. J Immunol 2017; 199: 3176–3186. doi: 10.4049/jimmunol.1700140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cárdenes N, Álvarez D, Sellarés J, et al. . Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res Ther 2018; 9: 257. doi: 10.1186/s13287-018-0970-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paxson JA, Gruntman A, Parkin CD, et al. . Age-dependent decline in mouse lung regeneration with loss of lung fibroblast clonogenicity and increased myofibroblastic differentiation. PLoS One 2011; 6: e23232. doi: 10.1371/journal.pone.0023232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanai H, Shteinberg A, Porat Z, et al. . Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients. Aging 2015; 7: 664–672. doi: 10.18632/aging.100807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Álvarez D, Cárdenes N, Sellarés J, et al. . IPF lung fibroblasts have a senescent phenotype. Am J Physiol Lung Cell Mol Physiol 2017; 313: L1164–L1173. doi: 10.1152/ajplung.00220.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehmann M, Hu Q, Hu Y, et al. . Chronic WNT/β-catenin signaling induces cellular senescence in lung epithelial cells. Cell Signal 2020; 70: 109588. doi: 10.1016/j.cellsig.2020.109588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu Y, Ng-Blichfeldt JP, Ota C, et al. . Wnt/β-catenin signaling is critical for regenerative potential of distal lung epithelial progenitor cells in homeostasis and emphysema. Stem Cells 2020; in press [ 10.1002/stem.3241]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Königshoff M, Kramer M, Balsara N, et al. . WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 2009; 119: 772–787. doi: 10.1172/JCI33950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angelidis I, Simon LM, Fernandez IE, et al. . An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat Commun 2019; 10: 963. doi: 10.1038/s41467-019-08831-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 2011; 378: 1949–1961. doi: 10.1016/S0140-6736(11)60052-4 [DOI] [PubMed] [Google Scholar]
- 45.Plataki M, Koutsopoulos AV, Darivianaki K, et al. . Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest 2005; 127: 266–274. doi: 10.1378/chest.127.1.266 [DOI] [PubMed] [Google Scholar]
- 46.Wagner DE, Bonenfant NR, Parsons CS, et al. . Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 2014; 35: 3281–3297. doi: 10.1016/j.biomaterials.2013.12.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chilosi M, Poletti V, Rossi A. The pathogenesis of COPD and IPF: distinct horns of the same devil? Respir Res 2012; 13: 3. doi: 10.1186/1465-9921-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawson WE, Crossno PF, Polosukhin VV, et al. . Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 2008; 294: L1119–L1126. doi: 10.1152/ajplung.00382.2007 [DOI] [PubMed] [Google Scholar]
- 49.Borok Z, Horie M, Flodby P, et al. . Grp78 loss in epithelial progenitors reveals an age-linked role for endoplasmic reticulum stress in pulmonary fibrosis. Am J Respir Crit Care Med 2020; 201: 198–211. doi: 10.1164/rccm.201902-0451OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maitra M, Cano CA, Garcia CK. Mutant surfactant A2 proteins associated with familial pulmonary fibrosis and lung cancer induce TGF-β1 secretion. Proc Natl Acad Sci USA 2012; 109: 21064–21069. doi: 10.1073/pnas.1217069110 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Yao C, Guan X, Carraro G, et al. . Senescence of alveolar stem cells drives progressive pulmonary fibrosis. bioRxiv 2019; preprint [ 10.1101/820175]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X, Xu H, Hou J, et al. . Epithelial cell senescence induces pulmonary fibrosis through Nanog-mediated fibroblast activation. Aging 2019; 12: 242–259. doi: 10.18632/aging.102613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calhoun C, Shivshankar P, Saker M, et al. . Senescent cells contribute to the physiological remodeling of aged lungs. J Gerontol A Biol Sci Med Sci 2016; 71: 153–160. doi: 10.1093/gerona/glu241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shivshankar P, Brampton C, Miyasato S, et al. . Caveolin-1 deficiency protects from pulmonary fibrosis by modulating epithelial cell senescence in mice. Am J Respir Cell Mol Biol 2012; 47: 28–36. doi: 10.1165/rcmb.2011-0349OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herrera J, Forster C, Pengo T, et al. . Registration of the extracellular matrix components constituting the fibroblastic focus in idiopathic pulmonary fibrosis. JCI Insight 2019; 4: e125185. doi: 10.1172/jci.insight.125185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minagawa S, Araya J, Numata T, et al. . Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2011; 300: L391–L401. doi: 10.1152/ajplung.00097.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reyfman PA, Walter JM, Joshi N, et al. . Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 2019; 199: 1517–1536. doi: 10.1164/rccm.201712-2410OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams TS, Schupp JC, Poli S, et al. . Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv 2020; 6: eaba1983. doi: 10.1126/sciadv.aba1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Habermann AC, Gutierrez AJ, Bui LT, et al. . Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv 2020; 6: eaba1972. doi: 10.1126/sciadv.aba1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baker DJ, Wijshake T, Tchkonia T, et al. . Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011; 479: 232–236. doi: 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grosse L, Wagner N, Emelyanov A, et al. . Defined p16High senescent cell types are indispensable for mouse healthspan. Cell Metab 2020; 32: 87–99. doi: 10.1016/j.cmet.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 62.de Mochel NR, Cheong KN, Cassandras M, et al. . Sentinel p16INK4a+ cells in the basement membrane form a reparative niche in the lung. bioRxiv 2020; preprint [ 10.1101/2020.06.10.142893]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baarsma HA, Skronska-Wasek W, Mutze K, et al. . Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J Exp Med 2017; 214: 143–163. doi: 10.1084/jem.20160675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnes PJ, Baker J, Donnelly LE. Cellular senescence as a mechanism and target in chronic lung diseases. Am J Respir Crit Care Med 2019; 200: 556–564. doi: 10.1164/rccm.201810-1975TR [DOI] [PubMed] [Google Scholar]
- 65.Vij N, Chandramani-Shivalingappa P, Van Westphal C, et al. . Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am J Physiol Cell Physiol 2018; 314: C73–C87. doi: 10.1152/ajpcell.00110.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuro-o M, Matsumura Y, Aizawa H, et al. . Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390: 45–51. doi: 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 67.Koike K, Kondo Y, Sekiya M, et al. . Complete lack of vitamin C intake generates pulmonary emphysema in senescence marker protein-30 knockout mice. Am J Physiol Lung Cell Mol Physiol 2010; 298: L784–L792. doi: 10.1152/ajplung.00256.2009 [DOI] [PubMed] [Google Scholar]
- 68.Uejima Y, Fukuchi Y, Nagase T, et al. . Influences of inhaled tobacco smoke on the senescence accelerated mouse (SAM). Eur Respir J 1990; 3: 1029–1036. [PubMed] [Google Scholar]
- 69.Suga T, Kurabayashi M, Sando Y, et al. . Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am J Respir Cell Mol Biol 2000; 22: 26–33. doi: 10.1165/ajrcmb.22.1.3554 [DOI] [PubMed] [Google Scholar]
- 70.Sato A, Hirai T, Imura A, et al. . Morphological mechanism of the development of pulmonary emphysema in klotho mice. Proc Natl Acad Sci USA 2007; 104: 2361–2365. doi: 10.1073/pnas.0607882104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calvi CL, Podowski M, D'Alessio FR, et al. . Critical transition in tissue homeostasis accompanies murine lung senescence. PLoS One 2011; 6: e20712. doi: 10.1371/journal.pone.0020712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou S, Wright JL, Liu J, et al. . Aging does not enhance experimental cigarette smoke-induced COPD in the mouse. PLoS One 2013; 8: e71410. doi: 10.1371/journal.pone.0071410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Savale L, Chaouat A, Bastuji-Garin S, et al. . Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 179: 566–571. doi: 10.1164/rccm.200809-1398OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amsellem V, Gary-Bobo G, Marcos E, et al. . Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011; 184: 1358–1366. doi: 10.1164/rccm.201105-0802OC [DOI] [PubMed] [Google Scholar]
- 75.Holz O, Zühlke I, Jaksztat E, et al. . Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur Respir J 2004; 24: 575–579. doi: 10.1183/09031936.04.00143703 [DOI] [PubMed] [Google Scholar]
- 76.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration 2010; 80: 59–70. doi: 10.1159/000268287 [DOI] [PubMed] [Google Scholar]
- 77.Ahmad T, Sundar IK, Tormos AM, et al. . Shelterin telomere protection protein 1 reduction causes telomere attrition and cellular senescence via Sirtuin 1 deacetylase in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2017; 56: 38–49. doi: 10.1165/rcmb.2016-0198OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baker JR, Vuppusetty C, Colley T, et al. . MicroRNA-570 is a novel regulator of cellular senescence and inflammaging. FASEB J 2019; 33: 1605–1616. doi: 10.1096/fj.201800965R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mikawa R, Suzuki Y, Baskoro H, et al. . Elimination of p19ARF-expressing cells protects against pulmonary emphysema in mice. Aging Cell 2018; 17: e12827. doi: 10.1111/acel.12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sundar IK, Rashid K, Gerloff J, et al. . Genetic ablation of p16INK4a does not protect against cellular senescence in mouse models of chronic obstructive pulmonary disease/emphysema. Am J Respir Cell Mol Biol 2018; 59: 189–199. doi: 10.1165/rcmb.2017-0390OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cottage CT, Peterson N, Kearley J, et al. . Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice. Commun Biol 2019; 2: 307. doi: 10.1038/s42003-019-0532-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chilosi M, Carloni A, Rossi A, et al. . Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl Res 2013; 162: 156–173. doi: 10.1016/j.trsl.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 83.Wu X, van Dijk EM, Ng-Blichfeldt JP, et al. . Mesenchymal WNT-5A/5B signaling represses lung alveolar epithelial progenitors. Cells 2019; 8: 1147. doi: 10.3390/cells8101147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takizawa H, Tanaka M, Takami K, et al. . Increased expression of transforming growth factor-β1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med 2001; 163: 1476–1483. doi: 10.1164/ajrccm.163.6.9908135 [DOI] [PubMed] [Google Scholar]
- 85.Morty RE, Königshoff M, Eickelberg O. Transforming growth factor-β signaling across ages. Proc Am Thorac Soc 2009; 6: 607–613. doi: 10.1513/pats.200908-087RM [DOI] [PubMed] [Google Scholar]
- 86.Pons AR, Sauleda J, Noguera A, et al. . Decreased macrophage release of TGF-β and TIMP-1 in chronic obstructive pulmonary disease. Eur Respir J 2005; 26: 60–66. doi: 10.1183/09031936.05.00045504 [DOI] [PubMed] [Google Scholar]
- 87.Zhang J, Wu L, Feng MX, et al. . Pulmonary fibroblasts from COPD patients show an impaired response of elastin synthesis to TGF-β1. Respir Physiol Neurobiol 2011; 177: 236–240. doi: 10.1016/j.resp.2011.04.019 [DOI] [PubMed] [Google Scholar]
- 88.Confalonieri M, Salton F, Fabiano F. Acute respiratory distress syndrome. Eur Respir Rev 2017; 26: 160116. doi: 10.1183/16000617.0116-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017; 377: 562–572. doi: 10.1056/NEJMra1608077 [DOI] [PubMed] [Google Scholar]
- 90.Bellani G, Laffey JG, Pham T, et al. . Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315: 788–800. doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 91.Phua J, Badia JR, Adhikari NK, et al. . Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med 2009; 179: 220–227. doi: 10.1164/rccm.200805-722OC [DOI] [PubMed] [Google Scholar]
- 92.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012; 122: 2731–2740. doi: 10.1172/JCI60331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suzuki Y, Cantu E, Christie JD. Primary graft dysfunction. Semin Respir Crit Care Med 2013; 34: 305–319. doi: 10.1055/s-0033-1348474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cardinal-Fernandez P, Lorente JA, Ballen-Barragan A, et al. . Acute respiratory distress syndrome and diffuse alveolar damage. New insights on a complex relationship. Ann Am Thorac Soc 2017; 14: 844–850. doi: 10.1513/AnnalsATS.201609-728PS [DOI] [PubMed] [Google Scholar]
- 95.Zhang B, Zhou X, Qiu Y, et al. . Clinical characteristics of 82 cases of death from COVID-19. PLos One 2020; 15: e0235458. doi: 10.1371/journal.pone.0235458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu Z, Shi L, Wang Y, et al. . Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8: 420–422. doi: 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tian S, Hu W, Niu L, et al. . Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020; 15: 700–704. doi: 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barton LM, Duval EJ, Stroberg E, et al. . COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol 2020; 153: 725–733. doi: 10.1093/ajcp/aqaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aguiar D, Lobrinus JA, Schibler M, et al. . Inside the lungs of COVID-19 disease. Int J Legal Med 2020; 134: 1271–1274. doi: 10.1007/s00414-020-02318-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herridge MS, Moss M, Hough CL, et al. . Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med 2016; 42: 725–738. doi: 10.1007/s00134-016-4321-8 [DOI] [PubMed] [Google Scholar]
- 101.Thille AW, Esteban A, Fernandez-Segoviano P, et al. . Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: a prospective cohort study of clinical autopsies. Lancet Respir Med 2013; 1: 395–401. doi: 10.1016/S2213-2600(13)70053-5 [DOI] [PubMed] [Google Scholar]
- 102.Huppert LA, Matthay MA. Alveolar fluid clearance in pathologically relevant conditions: in vitro and in vivo models of acute respiratory distress syndrome. Front Immunol 2017; 8: 371. doi: 10.3389/fimmu.2017.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gonzalez-Lopez A, Albaiceta GM. Repair after acute lung injury: molecular mechanisms and therapeutic opportunities. Crit Care 2012; 16: 209. doi: 10.1186/cc11224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davey A, McAuley DF, O'Kane CM. Matrix metalloproteinases in acute lung injury: mediators of injury and drivers of repair. Eur Respir J 2011; 38: 959–970. doi: 10.1183/09031936.00032111 [DOI] [PubMed] [Google Scholar]
- 105.Yusen RD, Edwards LB, Kucheryavaya AY, et al. . The Registry of the International Society for Heart and Lung Transplantation: thirty-second official adult lung and heart-lung transplantation report – 2015; focus theme: early graft failure. J Heart Lung Transplant 2015; 34: 1264–1277. doi: 10.1016/j.healun.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 106.De Santis MM, Bölükbas DA, Lindstedt S, et al. . How to build a lung: latest advances and emerging themes in lung bioengineering. Eur Respir J 2018; 52: 1601355. doi: 10.1183/13993003.01355-2016 [DOI] [PubMed] [Google Scholar]
- 107.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011; 32: 3233–3243. doi: 10.1016/j.biomaterials.2011.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gilpin SE, Charest JM, Ren X, et al. . Regenerative potential of human airway stem cells in lung epithelial engineering. Biomaterials 2016; 108: 111–119. doi: 10.1016/j.biomaterials.2016.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ren X, Moser PT, Gilpin SE, et al. . Engineering pulmonary vasculature in decellularized rat and human lungs. Nat Biotechnol 2015; 33: 1097–1102. doi: 10.1038/nbt.3354 [DOI] [PubMed] [Google Scholar]
- 110.Butler CR, Hynds RE, Gowers KHC, et al. . Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med 2016; 194: 156–168. doi: 10.1164/rccm.201507-1414OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nichols JE, La Francesca S, Niles JA, et al. . Production and transplantation of bioengineered lung into a large-animal model. Sci Transl Med 2018; 10: eaao3926. doi: 10.1126/scitranslmed.aao3926 [DOI] [PubMed] [Google Scholar]
- 112.Zhou H, Kitano K, Ren X, et al. . Bioengineering human lung grafts on porcine matrix. Ann Surg 2018; 267: 590–598. doi: 10.1097/SLA.0000000000002129 [DOI] [PubMed] [Google Scholar]
- 113.Bölükbas DA, Da Silva IAN, Rydell-Törmänen K, et al. . Preclinical evidence for the role of stem/stromal cells in COPD. In: Burgess JK, Heijink IH, eds. Stem Cell-Based Therapy for Lung Disease. Cham, Springer, 2019; pp. 73–96. [Google Scholar]
- 114.Cruz T, Rojas M. Preclinical evidence for the role of stem/stromal cells in targeting ARDS. In: Burgess JK, Heijink IH, eds. Stem Cell-Based Therapy for Lung Disease. Cham, Springer, 2019; pp. 199–217. [Google Scholar]
- 115.Cruz FF, Rocco PRM. The potential of factors released from mesenchymal stromal cells as therapeutic agents in the lung. In: Burgess JK, Heijink IH, eds. Stem Cell-Based Therapy for Lung Disease. Cham, Springer, 2019; pp. 57–70. [Google Scholar]
- 116.Patete CL, Toonkel RL, Glassberg M. Stem cell based therapy for lung disease Preclinical evidence for the role of stem/stromal cells Clinical application of stem/stromal cells in lung fibrosis. In: Burgess JK, Heijink IH, eds. Stem Cell-Based Therapy for Lung Disease. Cham, Springer, 2019; pp. 119–130. [Google Scholar]
- 117.Rolandsson Enes S, Weiss DJ. Cell therapy for lung disease: current status and future prospects. Curr Stem Cell Rep 2020; 6: 30–39. doi: 10.1007/s40778-020-00171-5 [DOI] [Google Scholar]
- 118.Ikonomou L, Wagner DE, Turner L, et al. . Translating basic research into safe and effective cell-based treatments for respiratory diseases. Ann Am Thorac Soc 2019; 16: 657–668. doi: 10.1513/AnnalsATS.201812-890CME [DOI] [PubMed] [Google Scholar]
- 119.Ryan AL, Ikonomou L, Atarod S, et al. . Stem cells, cell therapies, and bioengineering in lung biology and diseases 2017. An official American Thoracic Society workshop report. Am J Respir Cell Mol Biol 2019; 61: 429–439. doi: 10.1165/rcmb.2019-0286ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matthay MA, Calfee CS, Zhuo H, et al. . Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med 2019; 7: 154–162. doi: 10.1016/S2213-2600(18)30418-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berical A, Lee RE, Randell SH, et al. . Challenges facing airway epithelial cell-based therapy for cystic fibrosis. Front Pharmacol 2019; 10: 74. doi: 10.3389/fphar.2019.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Serrano-Mollar A, Gay-Jordi G, Guillamat-Prats R, et al. . Safety and tolerability of alveolar type II cell transplantation in idiopathic pulmonary fibrosis. Chest 2016; 150: 533–543. doi: 10.1016/j.chest.2016.03.021 [DOI] [PubMed] [Google Scholar]
- 123.Ma Q, Ma Y, Dai X, et al. . Regeneration of functional alveoli by adult human SOX9+ airway basal cell transplantation. Protein Cell 2018; 9: 267–282. doi: 10.1007/s13238-018-0506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Asmani M, Velumani S, Li Y, et al. . Fibrotic microtissue array to predict anti-fibrosis drug efficacy. Nat Commun 2018; 9: 2066. doi: 10.1038/s41467-018-04336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zscheppang K, Berg J, Hedtrich S, et al. . Human pulmonary 3D models for translational research. Biotechnol J 2018; 13: 1700341. doi: 10.1002/biot.201700341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barkauskas CE, Chung MI, Fioret B, et al. . Lung organoids: current uses and future promise. Development 2017; 144: 986–997. doi: 10.1242/dev.140103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McCauley KB, Hawkins F, Serra M, et al. . Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell 2017; 20: 844–857. doi: 10.1016/j.stem.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Strikoudis A, Cieślak A, Loffredo L, et al. . Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Rep 2019; 27: 3709–3723. doi: 10.1016/j.celrep.2019.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rock JR, Onaitis MW, Rawlins EL, et al. . Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 2009; 106: 12771–12775. doi: 10.1073/pnas.0906850106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hild M, Jaffe AB. Production of 3-D airway organoids from primary human airway basal cells and their use in high-throughput screening. Curr Protoc Stem Cell Biol 2016; 37: IE.9.1–IE.9.15. doi: 10.1002/cpsc.1 [DOI] [PubMed] [Google Scholar]
- 131.Tan Q, Choi KM, Sicard D, et al. . Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials 2017; 113: 118–132. doi: 10.1016/j.biomaterials.2016.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen H, Matsumoto K, Brockway BL, et al. . Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells 2012; 30: 1948–1960. doi: 10.1002/stem.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee JH, Bhang DH, Beede A, et al. . Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 2014; 156: 440–455. doi: 10.1016/j.cell.2013.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee JH, Tammela T, Hofree M, et al. . Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell 2017; 170: 1149–1163. doi: 10.1016/j.cell.2017.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McQualter JL, Yuen K, Williams B, et al. . Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA 2010; 107: 1414–1419. doi: 10.1073/pnas.0909207107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alder JK, Barkauskas CE, Limjunyawong N, et al. . Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci USA 2015; 112: 5099–5104. doi: 10.1073/pnas.1504780112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ng-Blichfeldt JP, de Jong T, Kortekaas RK, et al. . TGF-β activation impairs fibroblast ability to support adult lung epithelial progenitor cell organoid formation. Am J Physiol Lung Cell Mol Physiol 2019; 317: L14–L28. doi: 10.1152/ajplung.00400.2018 [DOI] [PubMed] [Google Scholar]
- 138.Ng-Blichfeldt JP, Schrik A, Kortekaas RK, et al. . Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation. EBioMedicine 2018; 36: 461–474. doi: 10.1016/j.ebiom.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gilpin SE, Wagner DE. Acellular human lung scaffolds to model lung disease and tissue regeneration. Eur Respir Rev 2018; 27: 180021. doi: 10.1183/16000617.0021-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.van der Velden JL, Wagner DE, Lahue KG, et al. . TGF-β1-induced deposition of provisional extracellular matrix by tracheal basal cells promotes epithelial-to-mesenchymal transition in a c-Jun NH2-terminal kinase-1-dependent manner. Am J Physiol Lung Cell Mol Physiol 2018; 314: L984–L997. doi: 10.1152/ajplung.00053.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wagner DE, Bonenfant NR, Sokocevic D, et al. . Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials 2014; 35: 2664–2679. doi: 10.1016/j.biomaterials.2013.11.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Booth AJ, Hadley R, Cornett AM, et al. . Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 2012; 186: 866–876. doi: 10.1164/rccm.201204-0754OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Godin LM, Sandri BJ, Wagner DE, et al. . Decreased laminin expression by human lung epithelial cells and fibroblasts cultured in acellular lung scaffolds from aged mice. PLoS One 2016; 11: e0150966. doi: 10.1371/journal.pone.0150966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Parker MW, Rossi D, Peterson M, et al. . Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 2014; 124: 1622–1635. doi: 10.1172/JCI71386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alsafadi HN, Uhl FE, Pineda RH, et al. . Applications and approaches for three-dimensional precision-cut lung slices. Disease modeling and drug discovery. Am J Respir Cell Mol Biol 2020; 62: 681–691. doi: 10.1165/rcmb.2019-0276TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martin C, Uhlig S, Ullrich V. Cytokine-induced bronchoconstriction in precision-cut lung slices is dependent upon cyclooxygenase-2 and thromboxane receptor activation. Am J Respir Cell Mol Biol 2001; 24: 139–145. doi: 10.1165/ajrcmb.24.2.3545 [DOI] [PubMed] [Google Scholar]
- 147.Akram KM, Yates LL, Mongey R, et al. . Live imaging of alveologenesis in precision-cut lung slices reveals dynamic epithelial cell behaviour. Nat Commun 2019; 10: 1178. doi: 10.1038/s41467-019-09067-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burgstaller G, Vierkotten S, Lindner M, et al. . Multidimensional immunolabeling and 4D time-lapse imaging of vital ex vivo lung tissue. Am J Physiol Lung Cell Mol Physiol 2015; 309: L323–L332. doi: 10.1152/ajplung.00061.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lehmann M, Buhl L, Alsafadi HN, et al. . Differential effects of nintedanib and pirfenidone on lung alveolar epithelial cell function in ex vivo murine and human lung tissue cultures of pulmonary fibrosis. Respir Res 2018; 19: 175. doi: 10.1186/s12931-018-0876-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Woodcock HV, Eley JD, Guillotin D, et al. . The mTORC1/4E-BP1 axis represents a critical signaling node during fibrogenesis. Nat Commun 2019; 10: 6. doi: 10.1038/s41467-018-07858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alsafadi HN, Staab-Weijnitz CA, Lehmann M, et al. . An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am J Physiol Lung Cell Mol Physiol 2017; 312: L896–L902. doi: 10.1152/ajplung.00084.2017 [DOI] [PubMed] [Google Scholar]
- 152.Uhl FE, Vierkotten S, Wagner DE, et al. . Preclinical validation and imaging of Wnt-induced repair in human 3D lung tissue cultures. Eur Respir J 2015; 46: 1150–1166. doi: 10.1183/09031936.00183214 [DOI] [PubMed] [Google Scholar]