FIGURE 1.
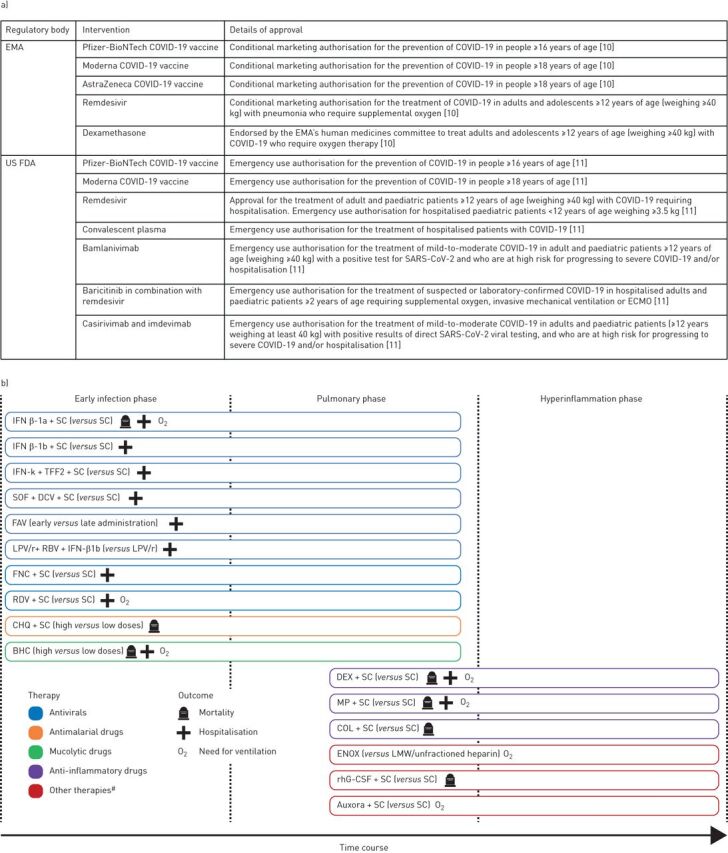
a) Current US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved interventions for coronavirus disease 2019 (COVID-19). b) Effective therapies in the included trials showing interventions that showed statistically significant differences in outcomes versus the comparator. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ECMO: extracorporeal membrane oxygenation; IFN: interferon; SC: standard care; TFF2: trefoil factor 2; SOF: sofosbuvir; DCV: daclatasvir; FAV: favipiravir; LPV/r: lopinavir/ritonavir; RBV: ribavirin; FNC: azvudine; RDV: remdesivir; CHQ: chloroquine diphosphate; BHC: bromhexine; DEX: dexamethasone; MP: methylprednisolone; COL: colchicine; ENOX: enoxaparin; LMW: low molecular weight; rhG-CSF: recombinant human granulocyte colony-stimulating factor. #: includes enoxaparin (anticoagulant), rhG-CSF and auxora (calcium release-activated calcium channel inhibitor).
